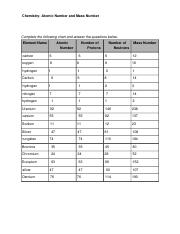
41 atomic mass and atomic number worksheet
Quiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element? Basic Atomic Structure Worksheet ANSWERS - Course Hero Basic Atomic Structure Worksheet ANSWERS 1 a) protons b) neutrons c) electrons a) Positive b) Neutral c) negative 2 atomic number (or identity); charge 3 protons; electrons (in a neutral charge atom only!); same 4 average atomic weight; mass 5 mass number; nucleus 6 neutrons; protons (or atomic number); mass number 7 Lithium = Li = 3 Bromine ...
Isotopes & Relative Atomic Mass (solutions, examples, videos) An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu). Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron) = mass of one carbon-12 atom = 1 amu (atomic mass unit) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson

Atomic mass and atomic number worksheet
Electron Configurations in Atomic Energy Levels - Study.com Aug 22, 2021 · The number of electrons in an atom's electron cloud is that element's atomic number. These electrons are arranged in specific energy levels surrounding the nucleus. Early Ideas about Matter | Chemistry | Visionlearning The module then describes how Lavoisier's Law of Conservation of Mass and Proust's Law of Definite Proportions contributed to Dalton's modern atomic theory. NGSS; HS-C5.1, HS-PS1.A3 ; Further Reading; States of Matter; Atomic Theory I; Anthony Carpi, Ph.D. “Early Ideas about Matter” Visionlearning Vol. CHE-1 (1), 2003. Atomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus.For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements.In an ordinary uncharged atom, the …
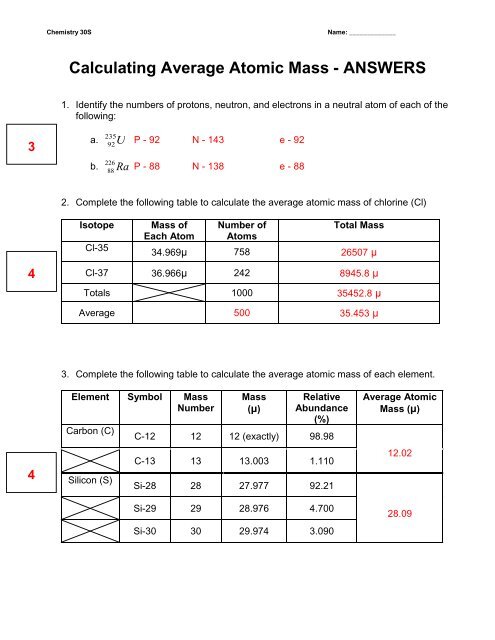
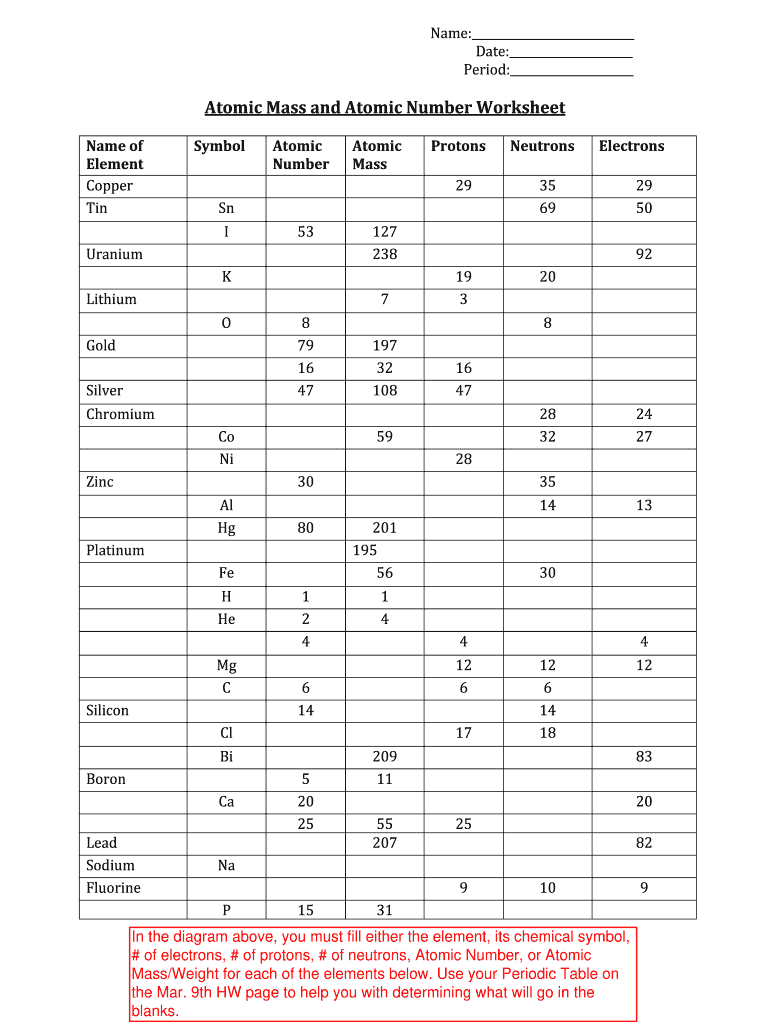
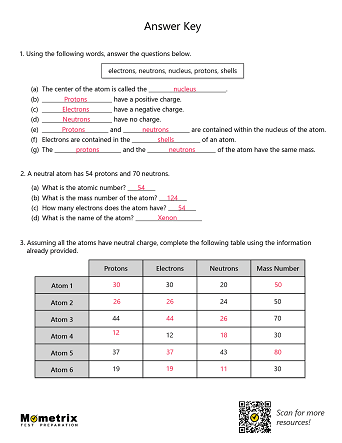
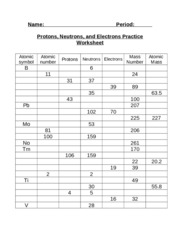
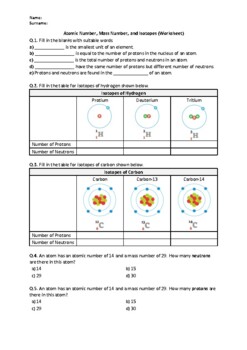
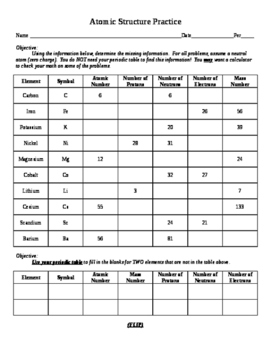
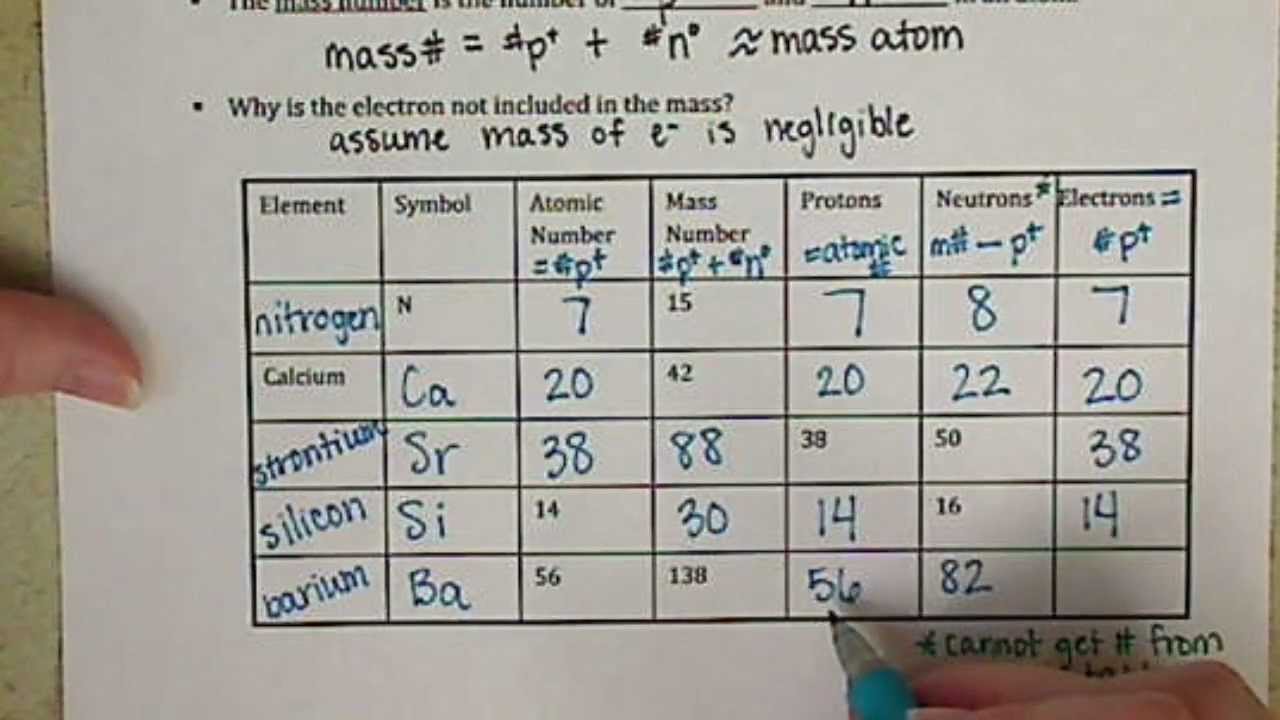
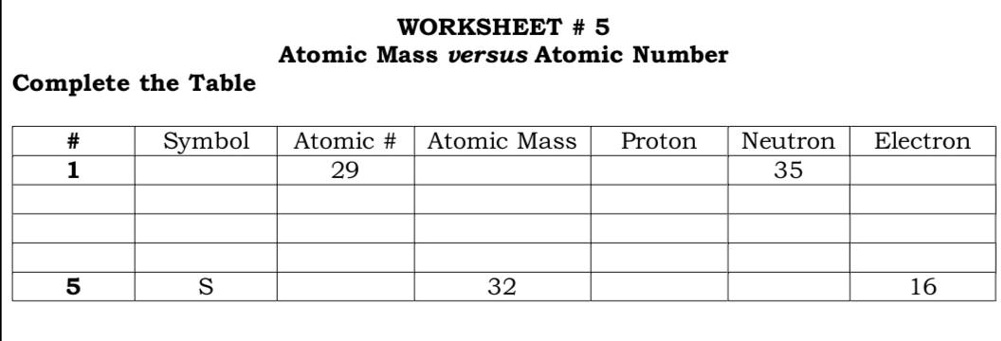
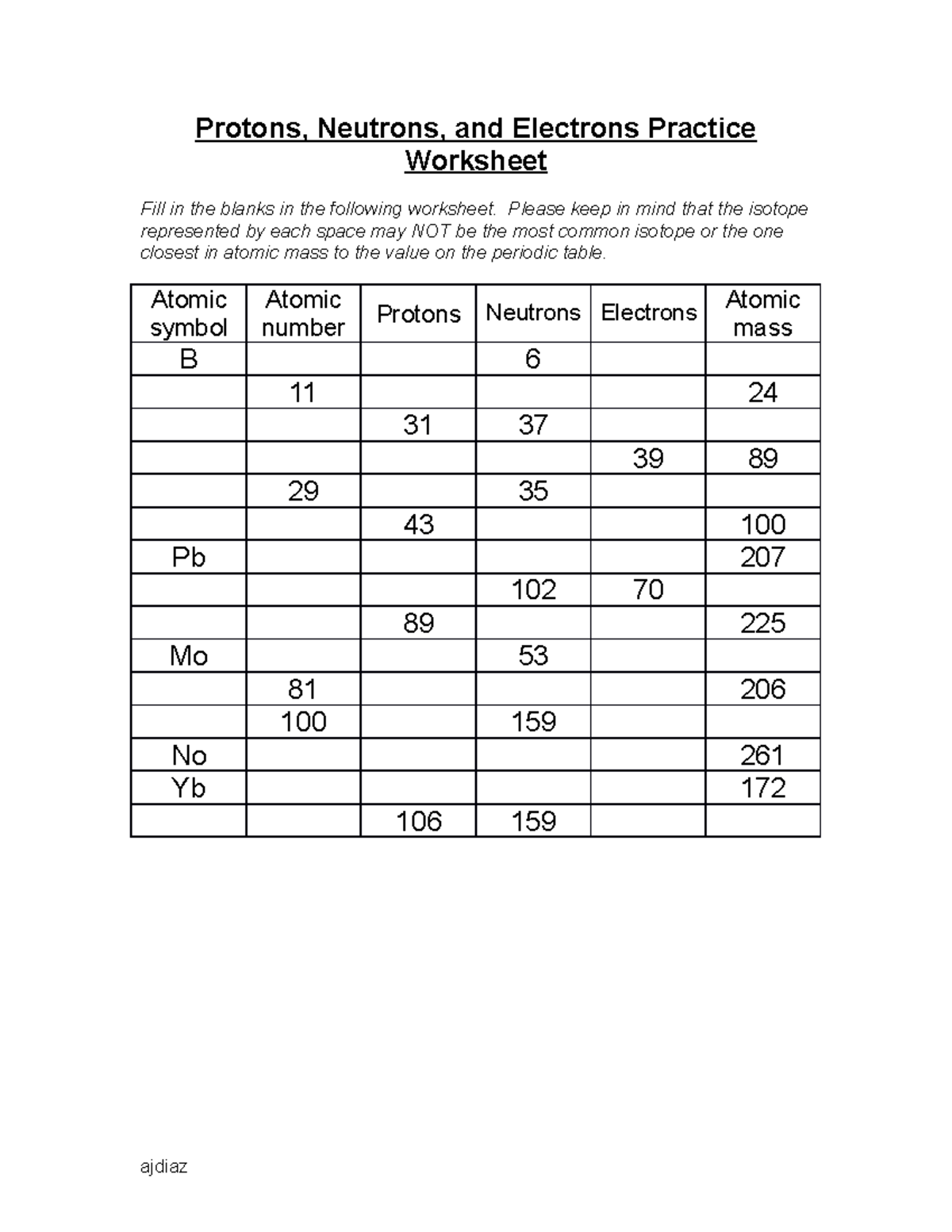
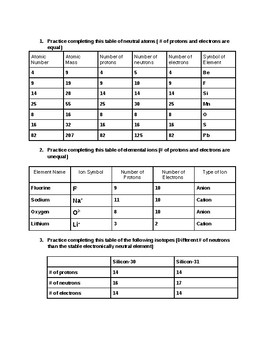
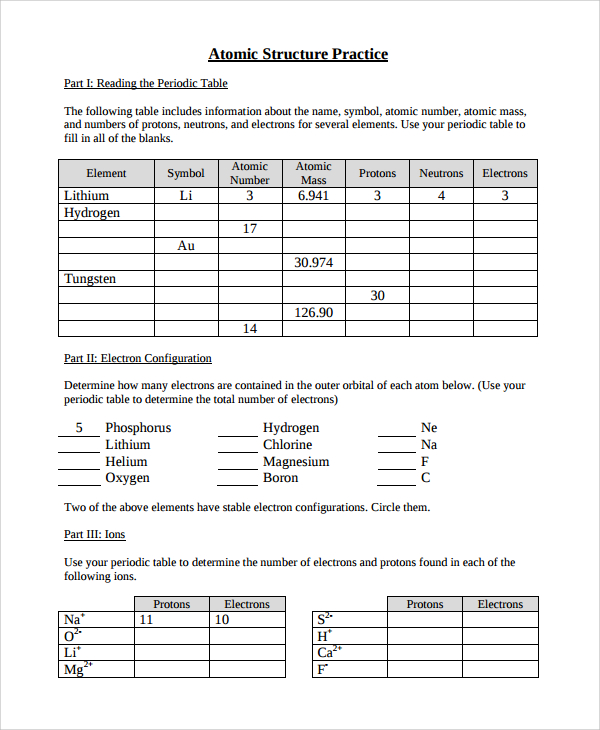
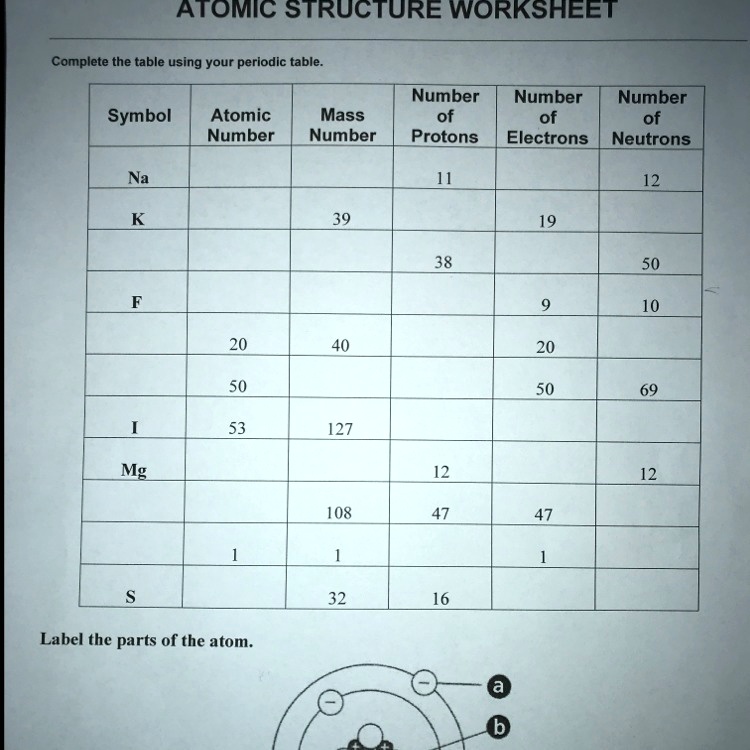
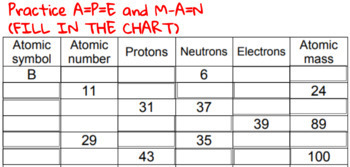
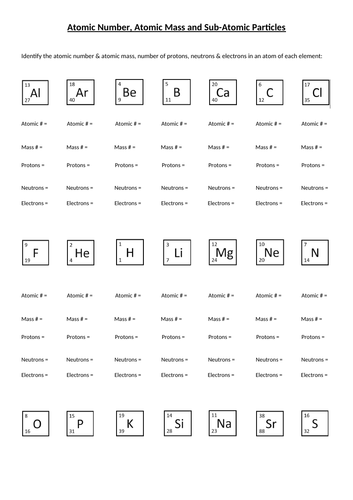
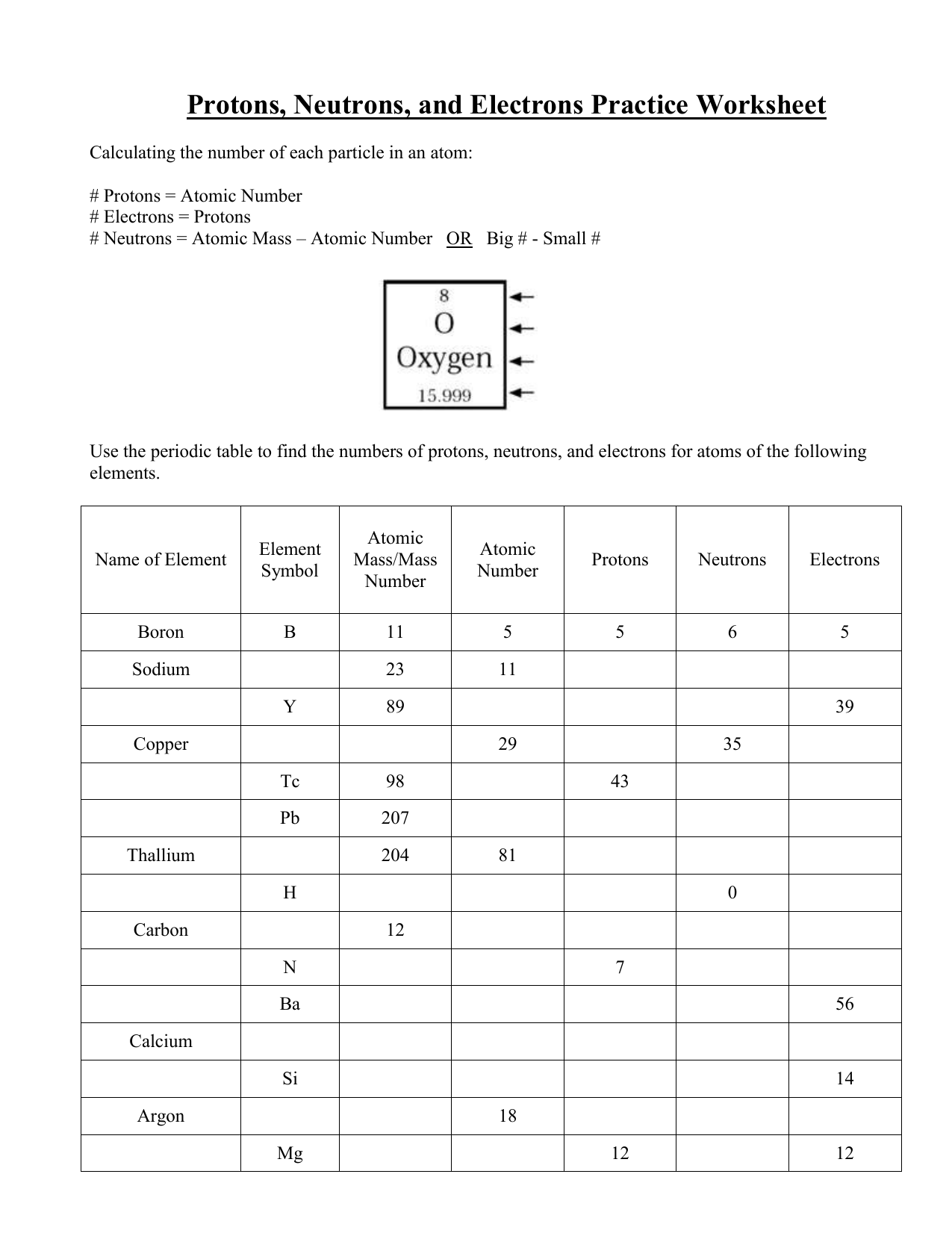
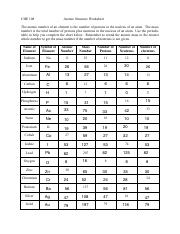
Atomic mass and atomic number worksheet. Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 Isotopes and Atomic Mass - PhET Isotopes and Atomic Mass - PhET Atomic Structure Worksheet - Washoe County School District Which particles account for the mass of the atom? (Atomic mass or mass number) and. 8.Com lete the followin table. Symbol Atomic Number Number of Protons Number of Neutrons Number of Electrons Mass 9 The atomic number is the number of in one atom of an element. It is also the number of in a neutral atom of that element. The atomic number gives ... Basic Atomic Structure Worksheet Key - Neshaminy School … have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In
Atomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus.For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements.In an ordinary uncharged atom, the … Early Ideas about Matter | Chemistry | Visionlearning The module then describes how Lavoisier's Law of Conservation of Mass and Proust's Law of Definite Proportions contributed to Dalton's modern atomic theory. NGSS; HS-C5.1, HS-PS1.A3 ; Further Reading; States of Matter; Atomic Theory I; Anthony Carpi, Ph.D. “Early Ideas about Matter” Visionlearning Vol. CHE-1 (1), 2003. Electron Configurations in Atomic Energy Levels - Study.com Aug 22, 2021 · The number of electrons in an atom's electron cloud is that element's atomic number. These electrons are arranged in specific energy levels surrounding the nucleus.




















0 Response to "41 atomic mass and atomic number worksheet"
Post a Comment