38 determining molecular formula worksheet
Determining Empirical Formula Worksheet Answers - coginspire Determining molecular formulas (true formulas) 1. Determine the empirical formula of a compound containing 20 23 aluminum and 79 77 chlorine. The percent composition (by mass) of each element in naphthalene is 93.7% carbon and 6.30% hydrogen. A compound with an empirical formula of c4h4o and a molar mass of 136 grams per mole. DOC Honors Chemistry I Problem Set #1 1. A sample is analyzed as containing 24.09 grams of potassium, 0.308 moles of manganese, and 7.42 x 1023 atoms of oxygen. What is the empirical formula? 24.09 g K x (1 mol / 39.098 g) = 0.61614 mol / .308 = 2 0.308 mol Mn = 0.308 mol / .308 = 1 7.42 x 1023 atoms O x (1 mol / 6.022 x 1023) = 1.232 mol O / .308 = 4
Empirical Formula And Molecular Formula Worksheet The Molecular Mass Is Found To Be 110.0 G/Mol.determine The Molecular Formula. Worksheet #8 empirical formulas 1. Percent composition and molecular formula worksheet 1. 4 (12 g/mol) + 4 (1 g/mol) + 1 (16 g/mol) = 68 g/mol. Empirical And Molecular Formulas Chemistry • 10Th Grade. Write the empirical formula for the following compounds. C=40% ...

Determining molecular formula worksheet
PDF 11. Empirical Formula Worksheet II EMPIRICAL AND MOLECULAR FORMULA WORKSHEET 1. An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical fomula. I $15 CX2Ô3 2. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen. Determine the compound's empirical formula. 3. PDF Practice Work 28: Empirical and Molecular Formulas The molecular mass of the compound is 58.12 g/mol. Determine the molecular formula. 8 min max PAGE 349, QUESTION 145 145) Determine the molecular formula for ibuprofen, a common headache remedy. Analysis of ibuprofen yields a molar mass of 206 g/mol and a percent composition of 75.7% C, 8.80% H, and 15.5% O. Determine the molecular formula. PDF Worksheet #8 Empirical Formulas H O N O 4I Determine the molecular formula for each compound whose percentage composition is shown below. 6. 84.9% Hg and the remainder Cl, with a molecular weight of 472.2 g/mol. 7. 12.26% N, 3.54% H, 28.1% S, and 56.1% O. The molecular weight is 228.2 g/mol. The formula is known to contain the NH 4 + grouping. Write your formula accordingly. 8.
Determining molecular formula worksheet. Molecular Formula Practice Test Questions - ThoughtCo The molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. This 10-question practice test deals with finding the molecular formula of chemical compounds.. A periodic table will be required to complete this test. Answers appear after the final question. PDF Empirical and Molecular Formulas Worksheet Empirical and Molecular Formulas Worksheet . Objectives: • be able to calculate empirical and molecular formulas . Empirical Formula . 1) What is the empirical formula of a compound that contains 0.783g of Carbon, 0.196g of Hydrogen and 0.521g of Oxygen? 2) What is empirical formula of a compound which consists of 89.14% Au and 10.80% of O? PDF Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet Key 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? C 3 H 3 O mass = 55 g/mole 2. If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? C 6 H 6 O 2 3. DOC Molecular Formulas Worksheet MOLECULAR FORMULAS WORKSHEET (Chemistry 11) A compound is composed of 7.20g of C, 1.20g of H, and 9.60g O. The molar mass of the compound is 180.g. Find the empirical and molecular formula for this compound. A compound is composed of 16.66g C and 3.49g H. The molar mass of the compound is 58g.
PDF Section 7.4 Determining Chemical Formulas - Weebly The steps in determining empirical and molecular formulas from percent composition or mass data are outlined below. As in other calculations, the route leads from mass through moles because formulas are based on the rel- ative numbers of moles of elements in each mole of compound. Express percent by mass In grams. Find the number of moles of Chem215-Engelhardt: KEY for Molecular (True) Formulas ... Determining molecular formula worksheet KEY for Molecular (True) Formulas Worksheet KEY for Formula Mass, Percent Compostion, & Mole Conversion Worksheet (posted Thursday, Jan. 19) Mole Study Guide--Chapter 10 Unit9RevWkANS Unit9ReviewProbs Empirical Formula Lab Data 2014 Hydrate Lab Student Data Topic 14 Topic 16 Topic 17 Topic 21 Topic 22 PDF Determining a Chemical Formula - Chino Valley Unified ... Determining a Chemical Formula Pre-Lab Discussion: In a compound, regardless of the size of the sample, the mass ratio between the atoms of that compound will always be the same. This mass ratio can be used to determine the formula, or atom ratio, of the compound. The problem is that the Empirical Formula Worksheets - DSoftSchools Some of the worksheets below are Empirical Formula Worksheets, several exam style questions like determine the empirical formula of a compound that contains 53.70% iron and 46.30% sulfur, … solutions are provided at the end of each worksheet. Once you find your worksheet (s), you can either click on the pop-out icon or download button to ...
Determining Empirical Formulas Worksheet Answers - Kayra Excel What is the molar mass and molecular formula of a nondissociating compound whose empirical formula is c4h2n , if 3. Some of the worksheets below are empirical formula worksheets, several exam style questions like determine the empirical formula of a compound that contains 53.70% iron and 46.30% sulfur,. Determining Empirical Formulas Worksheet Answers ... Worksheet 10 Determining Empirical Formulas Name what is the empirical formula lowest whole number ratio of the compounds below 1. Write the molecular formulas of the following compounds. 75 carbon 25 hydrogen as H D Smel 2. Answer key is in my RED binder if you would like to check your answers. 6 empirical mass 3003 Step 2. PDF Empirical and Molecular Formula Worksheet Empirical and Molecular Formula Worksheet. SHOW WORK ON A SEPARATE SHEET OF PAPER. Write the empirical formula for the following compounds. 1) C. 6H6 2) C8H18 3) WO. 2 4) C2H6O2 5) X39Y13 6) A compound with an empirical formula of C. 2. OH. 4. and a molar mass of 88 grams per mole. What is the molecular formula of this compound? PDF Determining Molecular Formulas Answers Determining Molecular Formulas (True Formulas) 1. The empirical formula of a compound is NO 2 Its molecular mass is 92 g/mol. What is its molecular formula? 2. The empirical formula of a compound is CH 2 Its molecular mass is 70 g/mol. What is its molecular formula? 3. A compound is found to be 40.0% carbon, 6.7% hydrogen and 53.5% oxygen.
PDF Determining Molecular Formulas (True Formulas) Determining Molecular Formulas (True Formulas) Directions: Solve the problems below. 1) The empirical formulas of a compound is NO 2. Its molar mass os 92 g/mol. What is its molecular formula? 2) The empirical formulas of a compound is CH 2. Its molecular mass is 70 g/mol. What is its ...
Empirical And Molecular Formula Worksheet 2 - Herbalied Determining Molecular Formula Worksheet Promotiontablecovers (assume percentages given in the problems are grams) step 1: Empirical and molecular formula worksheet 2. Convert to moles step 2. Empirical and molecular formula worksheet show work on a separate sheet of paper. Find the molecular formula of a compound that contains 30.45 % nitrogen ...
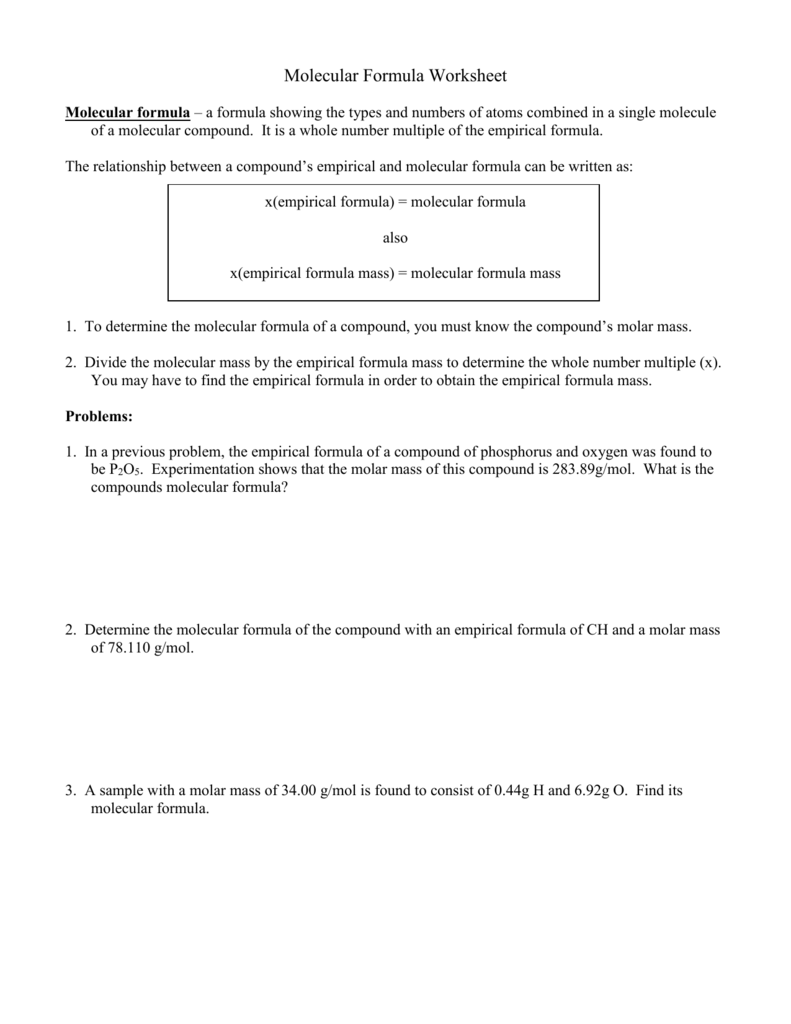
DOC Molecular Formula Worksheet - Livingston Public Schools Determine the molecular formula of a compound with an empirical formula of NH2 and a formula mass of 32.06 g/mol. 1. Empirical Formula = P2O5 Empirical Mass = 2(31.0) + 5(16.0) = 142g 283.89 ( 2 142 Molecular Mass = 283.89g. 2(P2O5) = P4O10 2. Empirical Formula = CH Empirical Mass = 1(12.0) + 1(1.0) = 13g
PDF Worksheet #8 Empirical Formulas H O N O 4I Determine the molecular formula for each compound whose percentage composition is shown below. 6. 84.9% Hg and the remainder Cl, with a molecular weight of 472.2 g/mol. 7. 12.26% N, 3.54% H, 28.1% S, and 56.1% O. The molecular weight is 228.2 g/mol. The formula is known to contain the NH 4 + grouping. Write your formula accordingly. 8.
PDF Practice Work 28: Empirical and Molecular Formulas The molecular mass of the compound is 58.12 g/mol. Determine the molecular formula. 8 min max PAGE 349, QUESTION 145 145) Determine the molecular formula for ibuprofen, a common headache remedy. Analysis of ibuprofen yields a molar mass of 206 g/mol and a percent composition of 75.7% C, 8.80% H, and 15.5% O. Determine the molecular formula.
PDF 11. Empirical Formula Worksheet II EMPIRICAL AND MOLECULAR FORMULA WORKSHEET 1. An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical fomula. I $15 CX2Ô3 2. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen. Determine the compound's empirical formula. 3.








0 Response to "38 determining molecular formula worksheet"
Post a Comment