39 mole to mole worksheet
Mass-Mole Conversions Worksheet Mass-Mole Conversions Worksheet. Molar mass of a substance = mass of one mole of the substance. One mole of an element = the atomic mass of that element ... CHEMISTRY: A Study of Matter - Weebly Title: Microsoft Word - 8-07a,b Mole-Mole Problems Wkst-Key.doc Author: HP_Administrator Created Date: 1/16/2008 4:09:36 PM
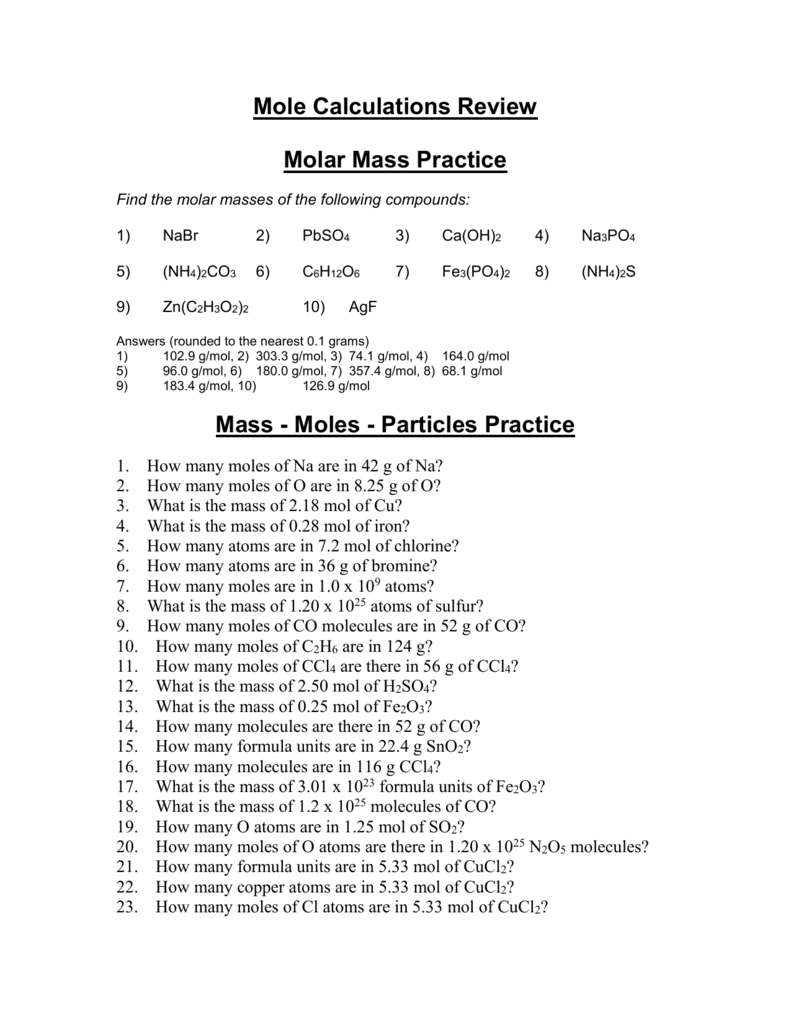
Mole Calculation Worksheet - Brookside High School Mole Calculation Worksheet – Answer Key What are the molecular weights of the following compounds? 1) NaOH 22.99 + 16.00 + 1.01 = 40.00 grams/mol 2) H 3 PO 4 3(1.01) + 30.97 + 4(16.00) = 98.00 grams

Mole to mole worksheet
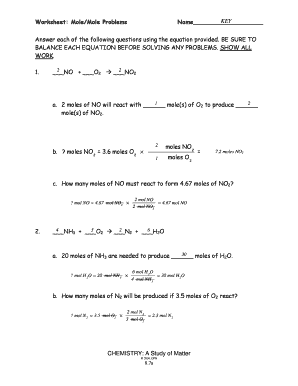
Mole Calculation Worksheet - Everett Community College Mole Calculation Worksheet W 340 ... 2314 mole Cd x 6.022 x 10 atoms Cd = 8.4 x 1023 atoms Cd 1 mole Cd 4) How many moles are in 4.3 x 1022 molecules of H 3 PO 4? Moles Worksheet - Science Classroom Teacher Resources Moles Worksheet (Solutions) 1) Define “mole”. 6.02 x 1023 of anything, usually atoms or molecules. 2) How many moles are present in 34 grams of Cu(OH)2? 0.35 moles 3) How many moles are present in 2.45 x 1023 molecules of CH 4? 0.41 moles 4) How many grams are there in 3.4 x 1024 molecules of NH 3? 96 grams 5) How much does 4.2 moles of Ca ... Stoichiometry: Mole-Mole Problems Stoichiometry: Mole-Mole Problems. N2 + 3H2 → 2NH3. How many moles of hydrogen, H2, are needed to react with 2.0 moles of nitrogen, N2? 2KClO3 → 2KCl + 3O2. How many moles of oxygen are produced by the decomposition of 6.0 moles of potassium chlorate, KClO3? Zn + 2HCl → ZnCl2 + H2
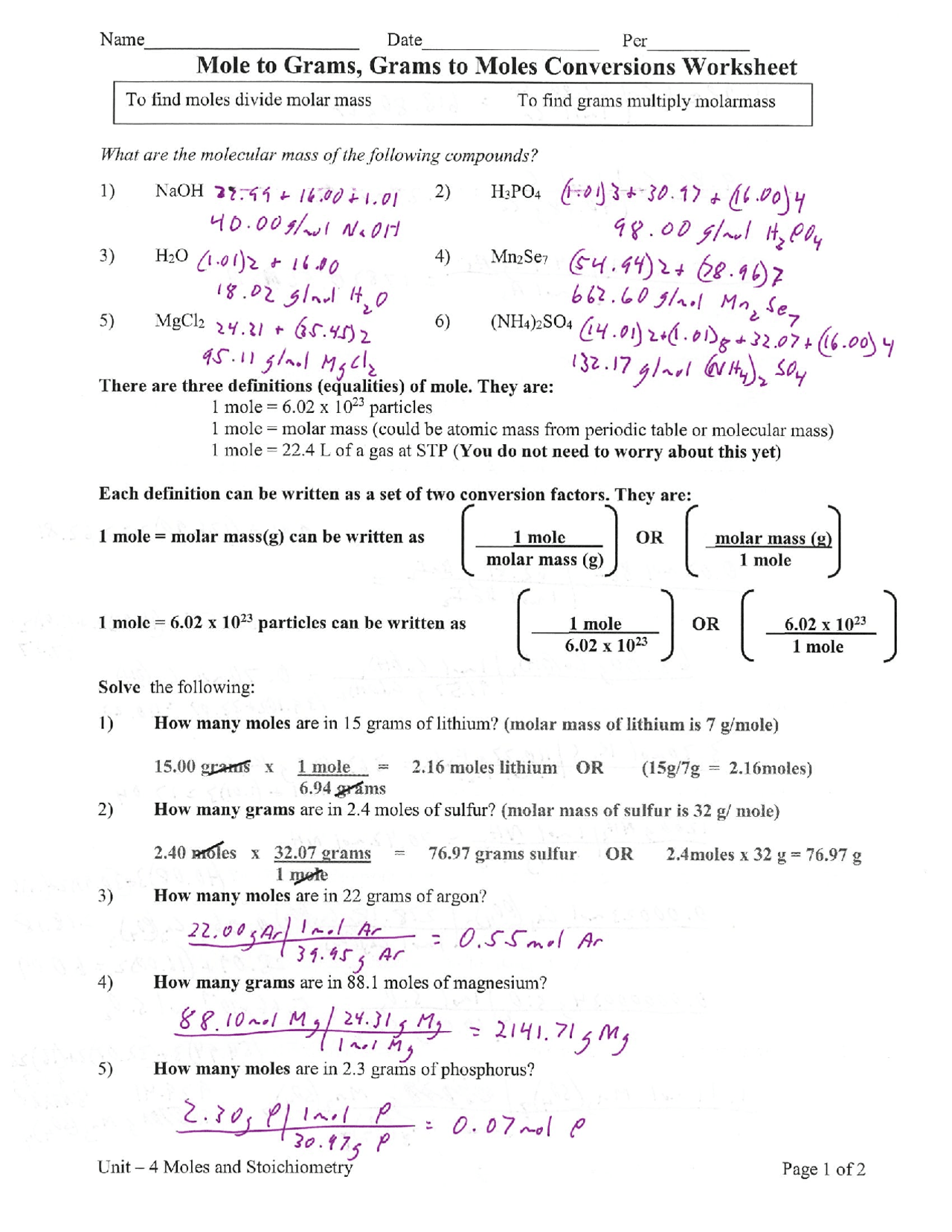
Mole to mole worksheet. Stoichiometry Worksheet - Socorro Independent School District Mole-Mole Stoichiometry Worksheet. 1. Given the following equation: 2 C4H10 + 13 O2 ---> 8 CO2 + 10 H2O . Show what the following molar ratios should be. Mole Conv WS KEY.pdf Mole Conversions Worksheet. There are three mole equalities. They are: 1 mol = 6.022 x 1023 particles. 1 mol = molar mass in grams (periodic table - red ... Stoichiometry: Mole-Mole Problems Stoichiometry: Mole-Mole Problems. N2 + 3H2 → 2NH3. How many moles of hydrogen, H2, are needed to react with 2.0 moles of nitrogen, N2? 2KClO3 → 2KCl + 3O2. How many moles of oxygen are produced by the decomposition of 6.0 moles of potassium chlorate, KClO3? Zn + 2HCl → ZnCl2 + H2 Moles Worksheet - Science Classroom Teacher Resources Moles Worksheet (Solutions) 1) Define “mole”. 6.02 x 1023 of anything, usually atoms or molecules. 2) How many moles are present in 34 grams of Cu(OH)2? 0.35 moles 3) How many moles are present in 2.45 x 1023 molecules of CH 4? 0.41 moles 4) How many grams are there in 3.4 x 1024 molecules of NH 3? 96 grams 5) How much does 4.2 moles of Ca ...
Mole Calculation Worksheet - Everett Community College Mole Calculation Worksheet W 340 ... 2314 mole Cd x 6.022 x 10 atoms Cd = 8.4 x 1023 atoms Cd 1 mole Cd 4) How many moles are in 4.3 x 1022 molecules of H 3 PO 4?






























0 Response to "39 mole to mole worksheet"
Post a Comment