40 section 10.3 percent composition and chemical formulas worksheet answers
Ch.10 - The Moles Mole: (working definition) the mass of a compound that is the same number of grams as the compound's formula or molecular mass in amu. Mole: (formal definition) the amount of matter that contains as many objects (atoms, molecules, etc.) as the number of atoms in exactly 12 g of 12C. Avogadro's constant: 1 mole = 6.02 x 1023 atoms, molecules, etc. Campbell Biology [12 ed.] 9780135188743, 9780135988046, 0135188741, 0136623441, 9780136623441. Campbell Biology, 12th Edition, delivers an authoritative, accurate ...
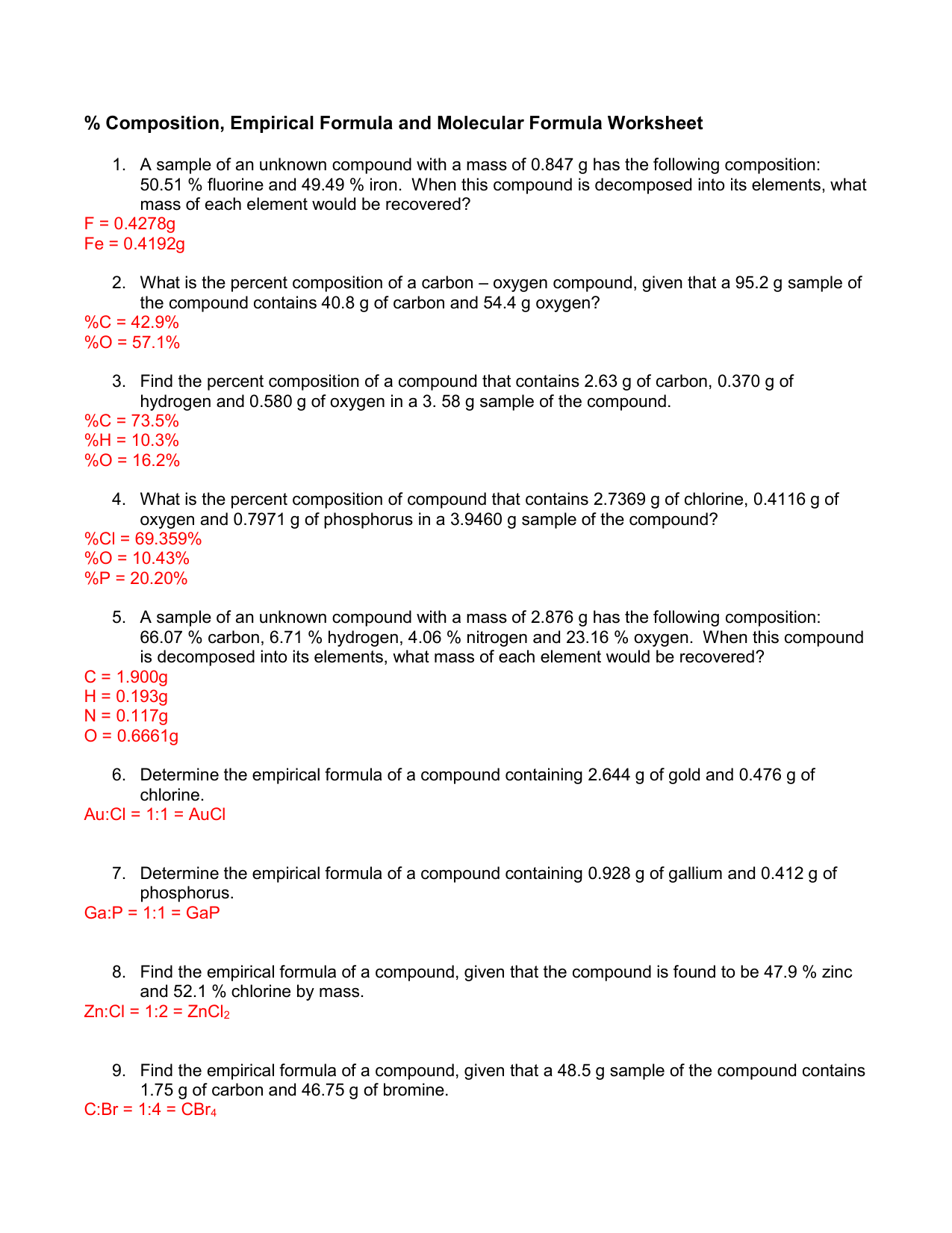
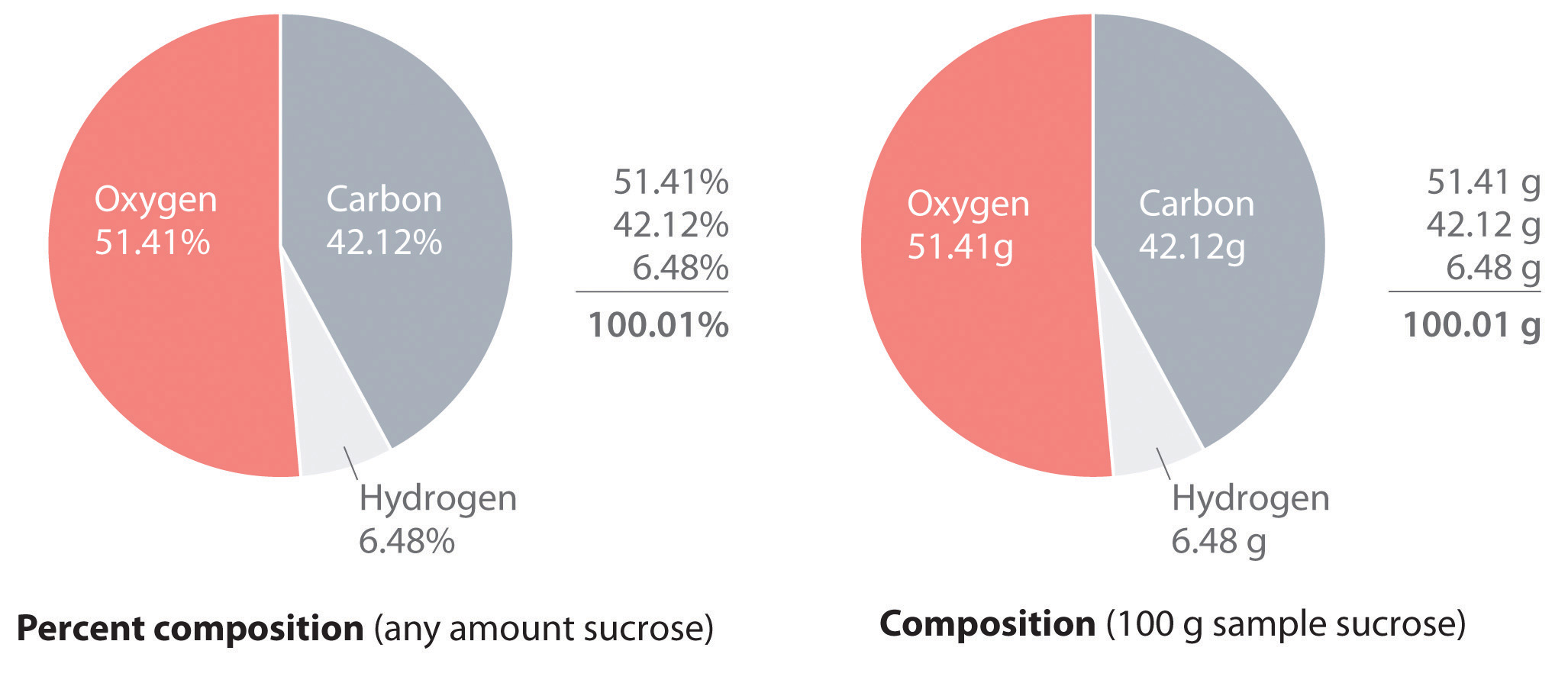
Section 10.3 Percent Composition and Chemical Formulas 305 10.3 Percent Composition and Chemical Formulas Is your shirt made of 100 percent cotton or wool, or is the fabric a combination of two or more fibers? A tag sewed into the seam of the shirt usually tells you what fibers were used to make the cloth and the percent of each.
Section 10.3 percent composition and chemical formulas worksheet answers
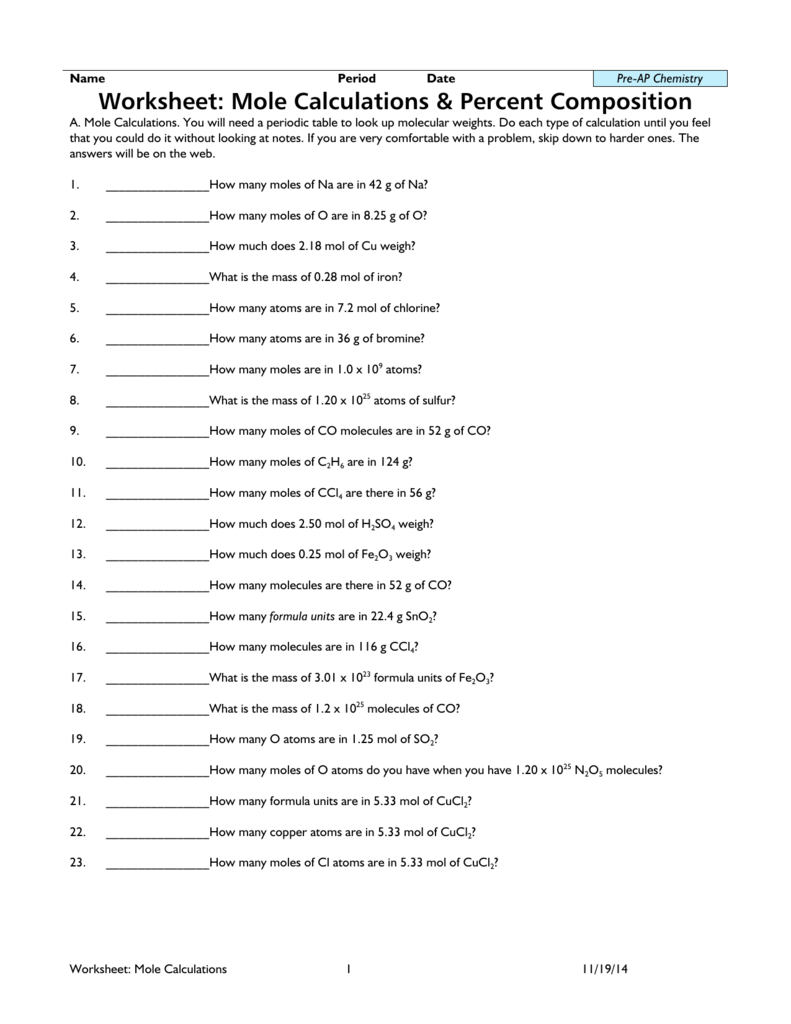
Holt Chemistry 1 The Mole and Chemical Composition Section: Avogadro's Number and Molar Conversions Solve the following problems, and write your answer in the space provided. 1. Determine the number of atoms present in 4.00 mol of aluminum. 2. Determine the number of atoms present in 1.55 mol of sodium. 3. Start studying Chapter 10.3 Percent Composition and Chemical Formulas. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Section 10.3 - Percent Composition and Chemical Formulas The percent by mass (percent composition) of an element in a compound is the number of grams of the element divided by the mass in grams of the compound multiplied by 100%. % mass of element = mass of element x 100 mass of compound Sample Problem
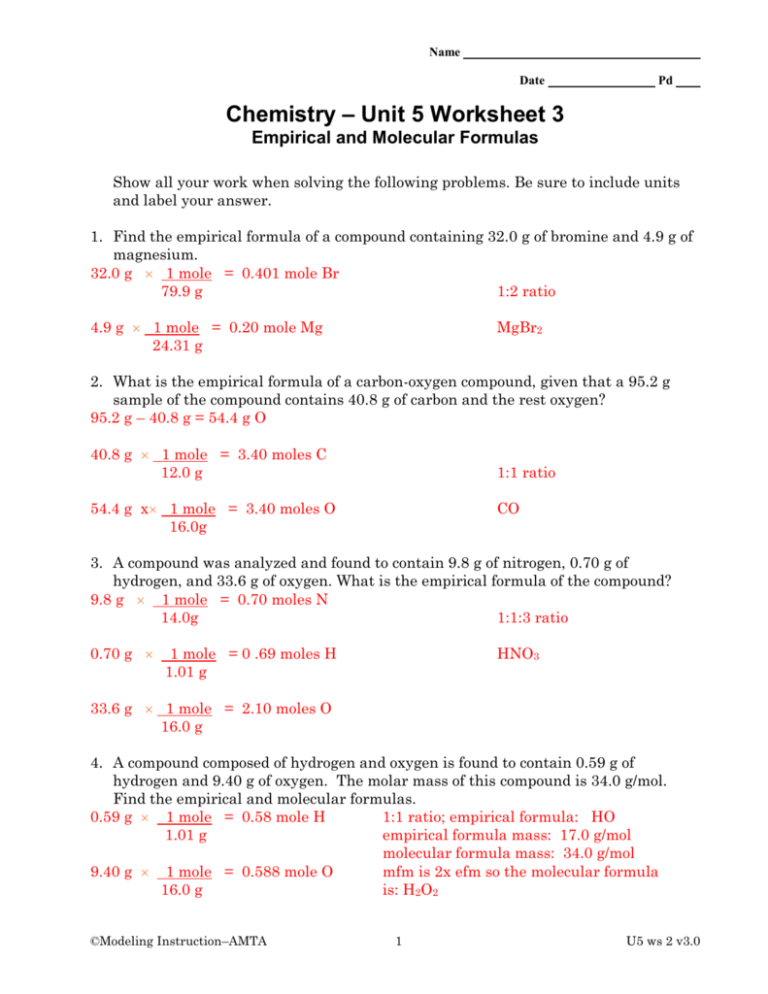
Section 10.3 percent composition and chemical formulas worksheet answers. 10.3: Percent Composition and Chemical Formulas STUDY PLAY percent composition -the percent by mass of each element in the compound -consists of a percent value for each element in the compound percent by mass of an element (mass of element)/(mass of compound) x 100% read the section "percent composition as a conversion factor" empirical formula Created Date: 2/13/2013 10:31:21 AM Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 45 Answer The molecular formula of a compound is either the same as its experimentally determined empirical formula, or it is a simple whole-number multiple of its empirical formula. Created Date: 2/3/2014 8:17:21 AM
2) Find the percent composition of calcium phosphate. 310-3 3) Find the percent composition of magnesium chromate. Croq - 4) A sample of iron (Ill) oxide has a mass of2.9g. After analysis, it was found to contain 1.7g of iron and I .2g of oxygen. Find the percent composition of this compound. 5) Determine the empirical formula of a compound ... Chapter 10 Chemical Quantities95 SECTION 10.3 PERCENT COMPOSITION AND CHEMICAL FORMULAS (pages 305-312) This section explains how to calculate percent composition from chemical formulas or experimental data, and how to derive empirical and molecular formulas. Percent Composition of a Compound (pages 305-308) 1. 10.3 Percent Composition and Chemical Formulas 15 > Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved.. You can also calculate the percent We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, master's or pHD, and we will assign you a writer who can satisfactorily meet your professor's expectations.
Chemistry (12th Edition) answers to Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - Sample Problem 10.9 - Page 326 33 including work step by step written by community members like you. 10.1 The Mole: A Measurement of Matter - Sample Problem 10.1. Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 48 Answer 313.6 Work Step by Step H= 124 g/mol Ca (CHO) = 158.1658 H= 124 x 4 = 496 496/158.1658 x100 =313.6 Update this answer! You can help us out by revising, improving and updating this answer. Update this answer Download Free Chapter 7 Ionic Compounds And Metals Worksheet Answers Chapter 1 Organic Compounds: Alkanes In these two ionic compounds, the charges Z + and Z - are the same, so the difference in lattice energy will depend upon R o. 10.3 PERCENT COMPOSiTION AND CHEMICAL FORMULAS]~ ~ ] ~ ~ ~ ~.sa j-Use this completion exercise to check your kllowledge of the terms and your understanding of the concepts introduced in section. Each blank can be completed with CL term. short phrase, or number. I"~'
10.3 Percent Composition & Chemical Formulas Answer Key/Answers - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Here are the answers for chemistry 10.3 Percent Composition & Chemical Formulas
Ensure you request for assistant if you can’t find the section. When you are done the system will automatically calculate for you the amount you are expected to pay for your order depending on the details you give such as subject area, number of pages, urgency, and academic level. After filling out the order form, you fill in the sign up details. This details will be used by our support …
10.3 Percent Composition and Chemical Formulas A molecular formula of a compound is a whole-number multiple of its empirical formula. Lesson Summary Percent Composition of a Compound Percent composition is the percent by mass of each element in a compound. To find the percent by mass of an element in a compound, use the formula: massof element
Section 10.3 - Percent Composition and Chemical Formulas. The percent by mass ... Chapter 10 - Chemical Quantities Access Free Chapter 10 Chemical Quantities Answers Chapter 10 Chemical Quantities Answers If you ally habit such a referred chapter 10 chemical quantities answers book that will present
FlexBook® Platform + CK-12 Overview
27.08.2019 · 23 Likes, 9 Comments - Rhiannon (@rhi_write) on Instagram: “Let’s talk about writing processes 😏 everyone’s so different and unique in how they write so I…”
Section 10.3 - Percent Composition and Chemical Formulas The percent by mass (percent composition) of an element in a compound is the number of grams of the element divided by the mass in grams of the compound multiplied by 100%. % mass of element = mass of element x 100 mass of compound Sample Problem
Start studying Chapter 10.3 Percent Composition and Chemical Formulas. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Holt Chemistry 1 The Mole and Chemical Composition Section: Avogadro's Number and Molar Conversions Solve the following problems, and write your answer in the space provided. 1. Determine the number of atoms present in 4.00 mol of aluminum. 2. Determine the number of atoms present in 1.55 mol of sodium. 3.


























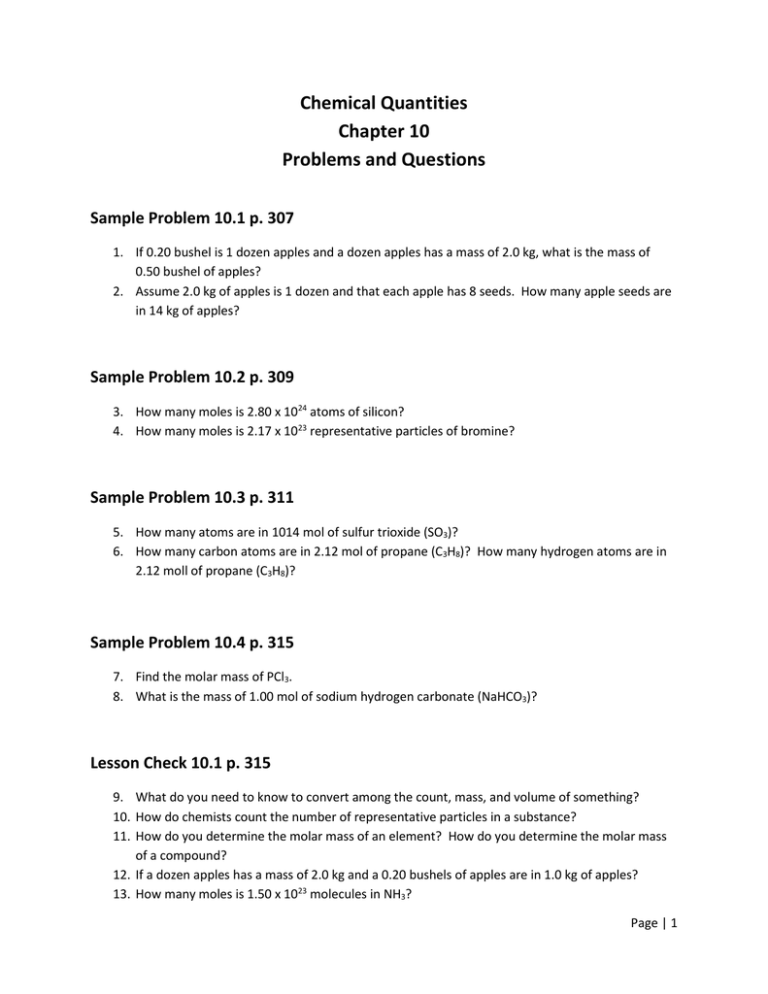

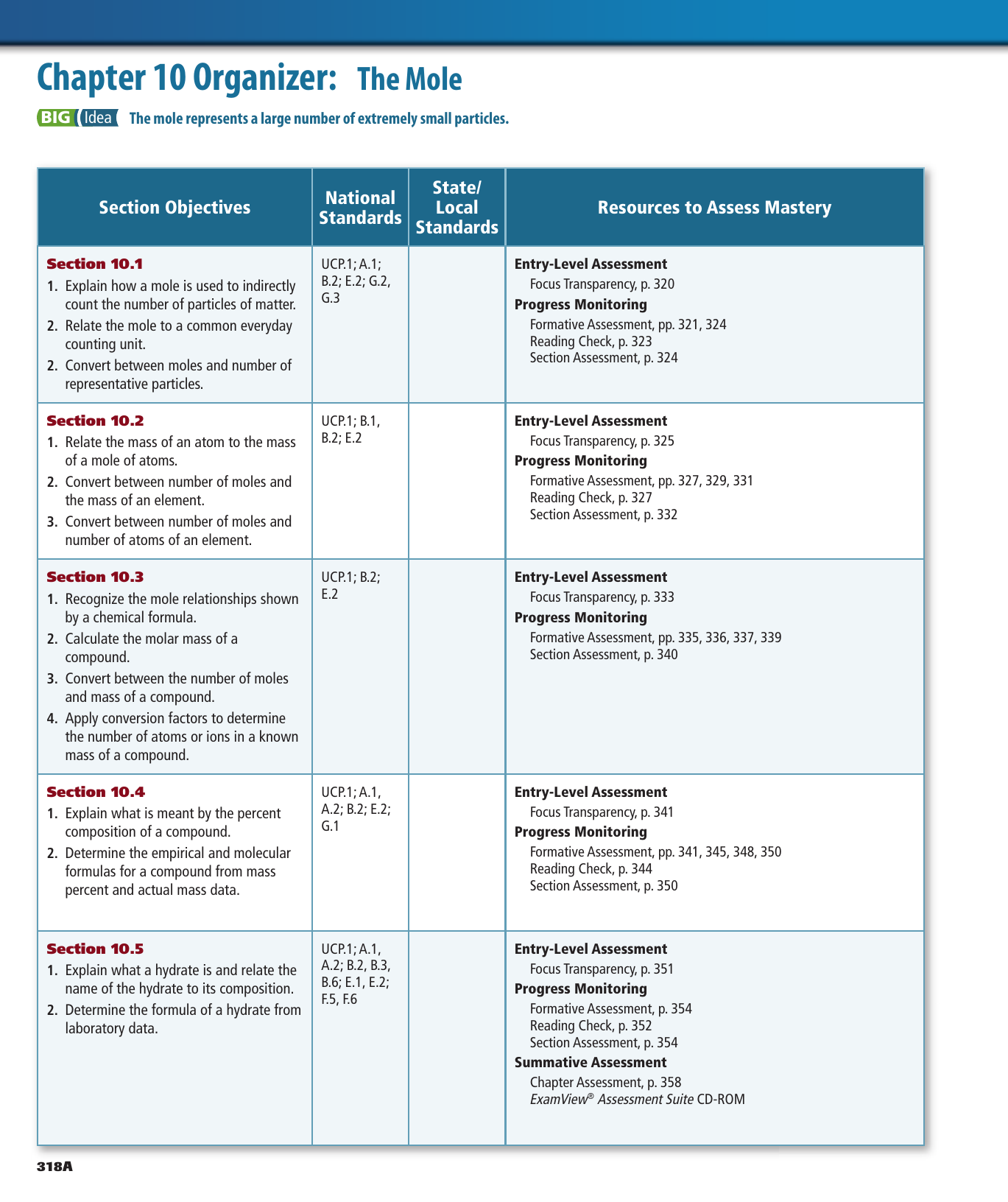


0 Response to "40 section 10.3 percent composition and chemical formulas worksheet answers"
Post a Comment