38 balancing nuclear reactions worksheet
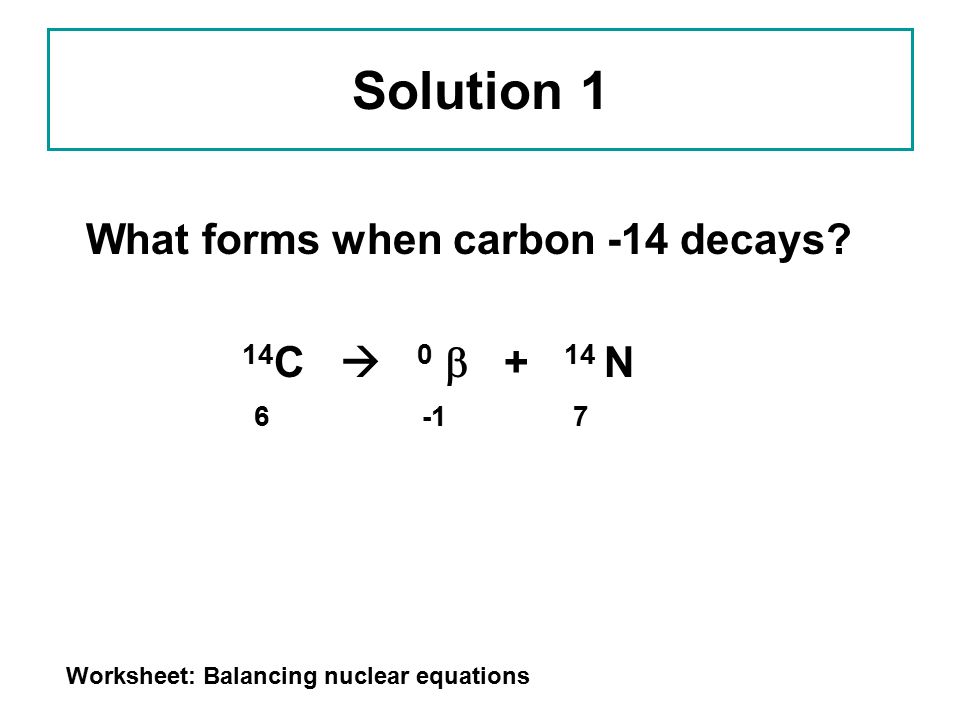
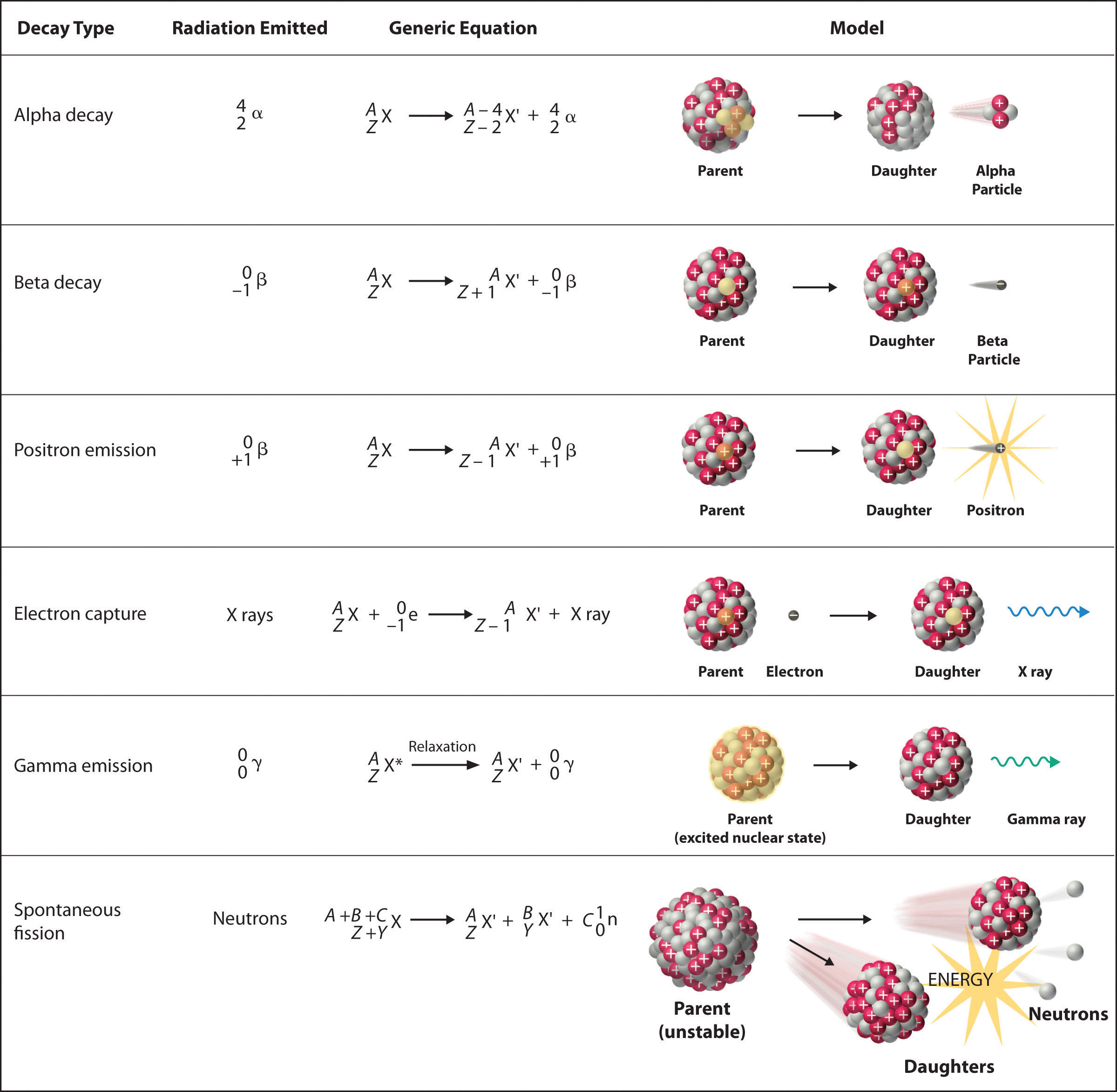
Balancing Nuclear Reactions Worksheet Answer Key Balancing Chemical Equations - Worksheet #3 Write balanced chemical equations for each of the following and then classify each reaction as a synthesis, decomposition, single-replacement, double replacement, acid-base reaction, or combustion. Be aware that some reactions may fall into more than one category. Balancing Nuclear Reactions Worksheet [jlk9xj227045] Balancing Nuclear Equations Name: Period: There are two types of nuclear reactions: Fission, where a nucleus breaks into two or more pieces, and fusion where two or more nuclei combine to form a new element. In nuclear reactions, only the nucleus is involved. Electrons are ignored.
Nuclear Reactions Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. Home Reload Open Download 2. Balancing Nuclear Equations - Reload Open Download 3. Home Reload Open Download 4. BALANCING NUCLEAR REACTIONS WORKSHEET Reload Open Download 5. Nuclear Chemistry Worksheet Reload Open Download 6. NUCLEAR REACTION WORKSHEET [ANSWER KEY] Reload Open Download 7.

Balancing nuclear reactions worksheet
PDF Loss of a - Fort Bend ISD • Nuclear reactions can change one element into another element 226Ra 88 222Rn 86 4 He 2 + Atomic number: 88 = 86 + 2 Mass number: 226 = 222 + 4 BALANCING A balanced nuclear reaction conserves the mass and atomic (proton) numbers. In other words, these values must be identical on both sides of the reaction X X = element symbol Z = atomic number Balancing Nuclear Reactions Worksheets - Lesson Worksheets Displaying all worksheets related to - Balancing Nuclear Reactions. Worksheets are Balancing nuclear reactions work, Unit 16 balancing nuclear reactions work, Unit 16 nuclear chemistry balancing nuclear reactions, Identifying nuclear reactions, Balancing equations practice problems, Math skills, Science balancing equations work, Balancing nuclear reactions practice work. 10++ Balancing Nuclear Equations Worksheet - Worksheets Decoomo 10++ Balancing Nuclear Equations Worksheet. A balanced nuclear response equation indicates that there's a rearrangement during a nuclear response but of subatomic particles quite than atoms. Balancing nuclear equations when balancing nuclear equations, the sums of the atomic and mass numbers must be the same on both sides of the equation.
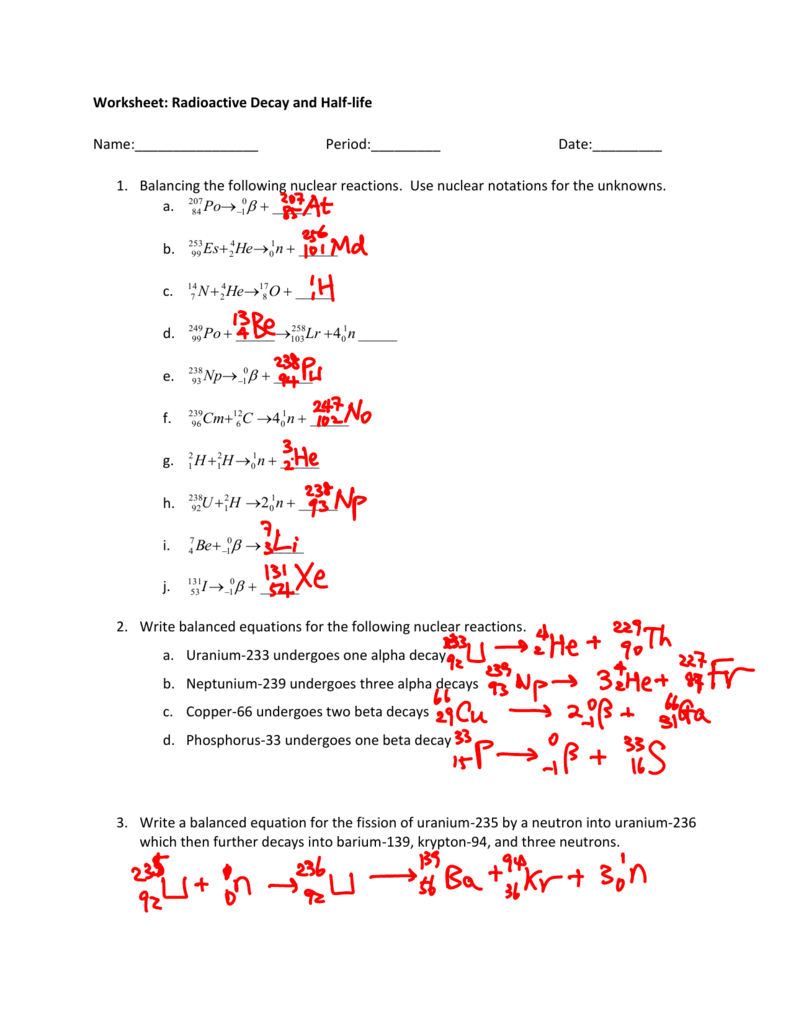
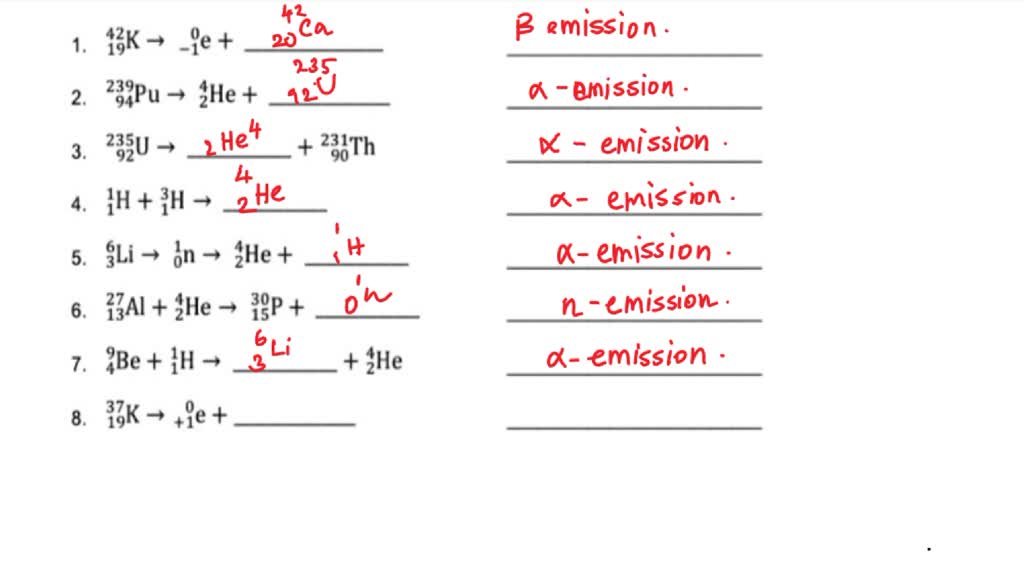
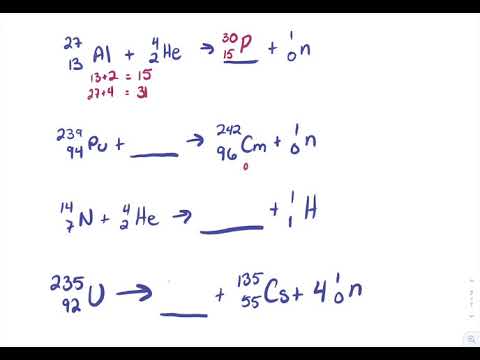
Balancing nuclear reactions worksheet. PDF BALANCING NUCLEAR REACTIONS WORKSHEET - eNetLearning BALANCING NUCLEAR REACTIONS WORKSHEET Predict the missing product or reactant in the following nuclear reactions. Determine the type of nuclear reaction (α emission, β emission, γ emission, positron emission, artificial ... HALF-LIFE PROBLEMS WORKSHEET 1.) What is the half-life of a 100.0 g sample of nitrogen-16 that decays to 12.5 grams in ... Lesson Worksheet:Balancing Nuclear Reactions | Nagwa In this worksheet, we will practice balancing equations for nuclear reactions and determining the nuclide symbols for unknown reactants and products. The portal has been deactivated. Please contact your portal admin. ... Lesson Worksheet: Balancing Nuclear Reactions Chemistry • 10th Grade ... PDF Balancing Nuclear Equations - Weebly Another type of reaction occurs when something impacts a nucleus. These reactions result either in the nucleus splitting (fission) or the combination of two or more nuclei to form a third, different nucleus (fusion). Balancing Nuclear Equations: Matter must be conserved including all p+ & n˚. Example: Decay reaction (α decay) 215! 86 219Rn" 2 ... Balancing Nuclear Reactions Worksheet - qstion.co Balancing nuclear reactions worksheet 1 when dissolved beryllium chloride reacts with dissolved silver nitrate in water aqueous beryllium. Determine the type of nuclear reaction (α emission, β emission, γ emission, positron emission, artificial transmutation, fission, or fusion) described. Write the complete nuclear equation.
PDF Nuclear Balance Worksheet Balancing Nuclear Equations When balancing nuclear equations, the sums of the atomic and mass numbers must be the same on both sides of the equation. In some cases one of the symbols in the list below will be used to complete the equation. If a new element is formed, you may need to refer to a DOC Balancing Nuclear Reactions Worksheet - Sch3u BALANCING NUCLEAR REACTIONS WORKSHEET Predict the missing product or reactant in the following nuclear reactions. Determine the type of nuclear reaction (α emission, β emission (+ or -) , γ emission, electron capture, other) Type of Nuclear Reaction 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. HALF-LIFE PROBLEMS WORKSHEET 1.) Balancing Nuclear Reactions Worksheet | PDF | Nuclear Reaction ... - Scribd These reactions result either in the nucleus splitting (fission) or the combination of two or more nuclei to form a third, different nucleus (fusion). Balancing Nuclear Equations: Matter must be conserved including all p+ & n. Example: Decay reaction ( decay) Fission Reaction Fusion Reaction: 219 86. Rn"24 He+ 215 84 Po. 1 0 35 17 Balancing Nuclear Reactions Worksheet - Chemistryworksheet.com Balancing Nuclear Reactions Worksheet is a free printable for you. This printable was uploaded at October 13, 2022 by tamble in Answers. Chemistry Challenge Worksheet Answers - If you're looking for Chemistry Worksheet Answers, you've come to the right place. {In this article, you'll learn more about the branches of chemistry, identifying ...
balancing_nuclear_reactions_worksheet.doc - BALANCING NUCLEAR REACTIONS ... BALANCING NUCLEAR REACTIONS WORKSHEET Predict the missing product or reactant in the following nuclear reactions. Determine the type of nuclear reaction (α emission, β emission (+ or -) , γ emission, electron capture, other) Type of Nuclear Reaction 1. Balancing Nuclear Reactions Worksheets - K12 Workbook Worksheets are Balancing nuclear reactions work, Unit 16 balancing nuclear reactions work, Unit 16 nuclear chemistry balancing nuclear reactions, Identifying nuclear reactions, Balancing equations practice problems, Math skills, Science balancing equations work, Balancing nuclear reactions practice work. 10++ Balancing Nuclear Equations Worksheet - Worksheets Decoomo 10++ Balancing Nuclear Equations Worksheet. A balanced nuclear response equation indicates that there's a rearrangement during a nuclear response but of subatomic particles quite than atoms. Balancing nuclear equations when balancing nuclear equations, the sums of the atomic and mass numbers must be the same on both sides of the equation. Balancing Nuclear Reactions Worksheets - Lesson Worksheets Displaying all worksheets related to - Balancing Nuclear Reactions. Worksheets are Balancing nuclear reactions work, Unit 16 balancing nuclear reactions work, Unit 16 nuclear chemistry balancing nuclear reactions, Identifying nuclear reactions, Balancing equations practice problems, Math skills, Science balancing equations work, Balancing nuclear reactions practice work.
PDF Loss of a - Fort Bend ISD • Nuclear reactions can change one element into another element 226Ra 88 222Rn 86 4 He 2 + Atomic number: 88 = 86 + 2 Mass number: 226 = 222 + 4 BALANCING A balanced nuclear reaction conserves the mass and atomic (proton) numbers. In other words, these values must be identical on both sides of the reaction X X = element symbol Z = atomic number






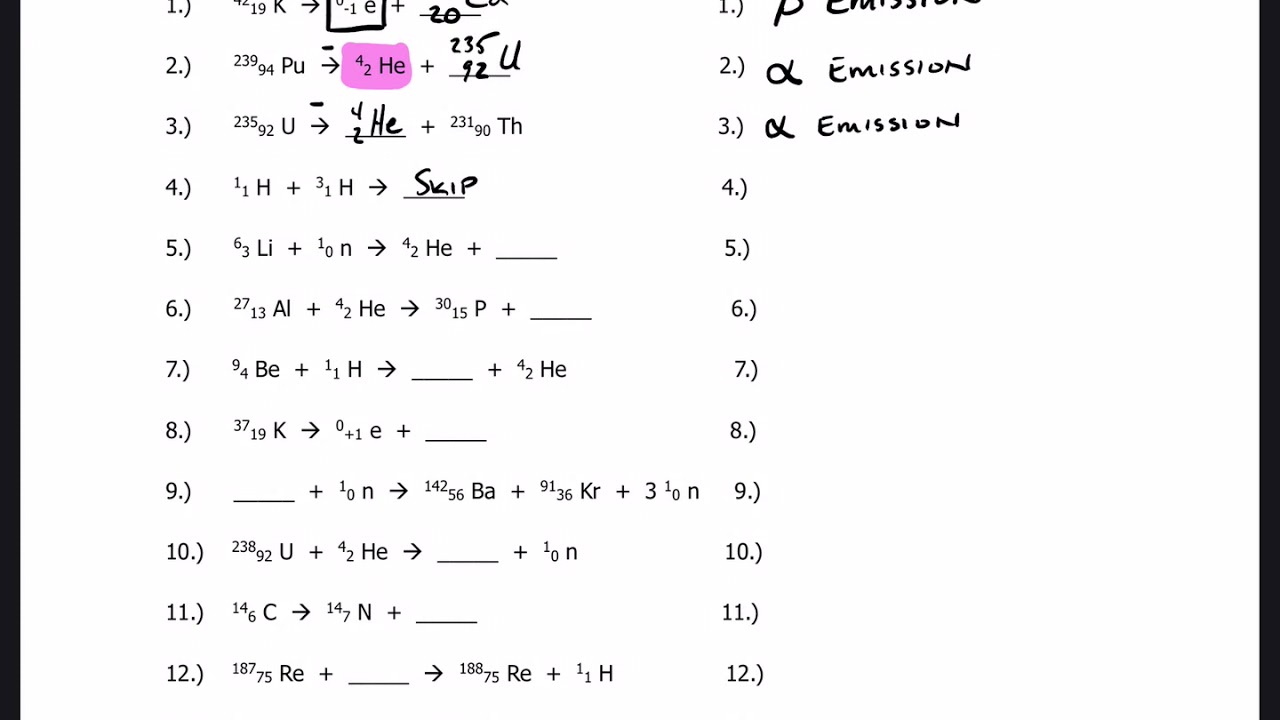












0 Response to "38 balancing nuclear reactions worksheet"
Post a Comment