44 protons neutrons and electrons practice worksheet
Build an Atom - Atoms | Atomic Structure | Isotope ... - PhET Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Atomic Structure & The Changing Models of Atom - BioChem … 24.9.2015 · A 11 protons and 12 neutrons. B 11 protons and 12 electrons. C 23 protons and 11 neutrons. D 23 protons and 11 electrons. Q23. The isotopes of magnesium, 24 12 Mg and 25 12 Mg, both form ions with charge 2+. Which of the following statements about these ions is true? A Both ions have electronic configuration 1s 2 2s 2 2p 6 3s 2.
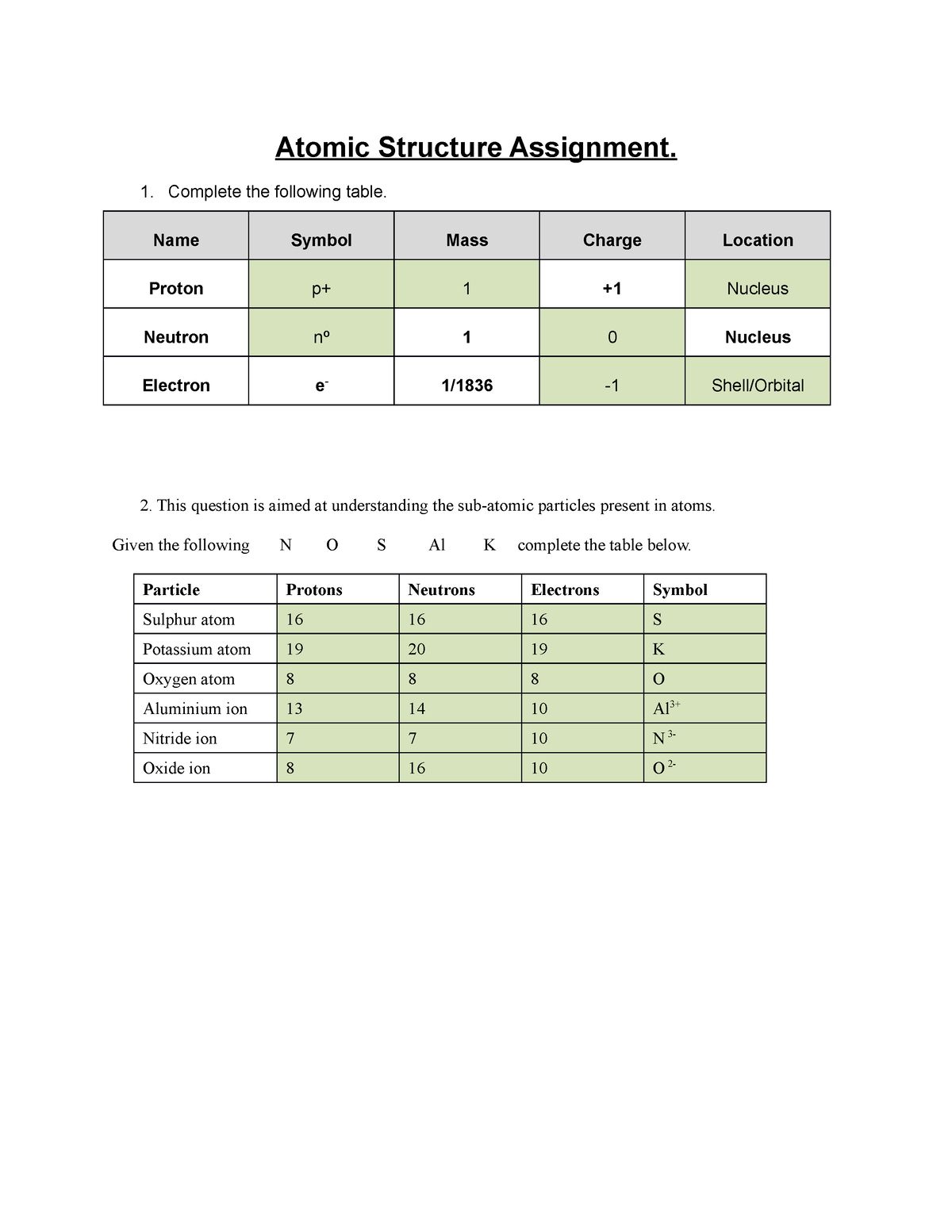
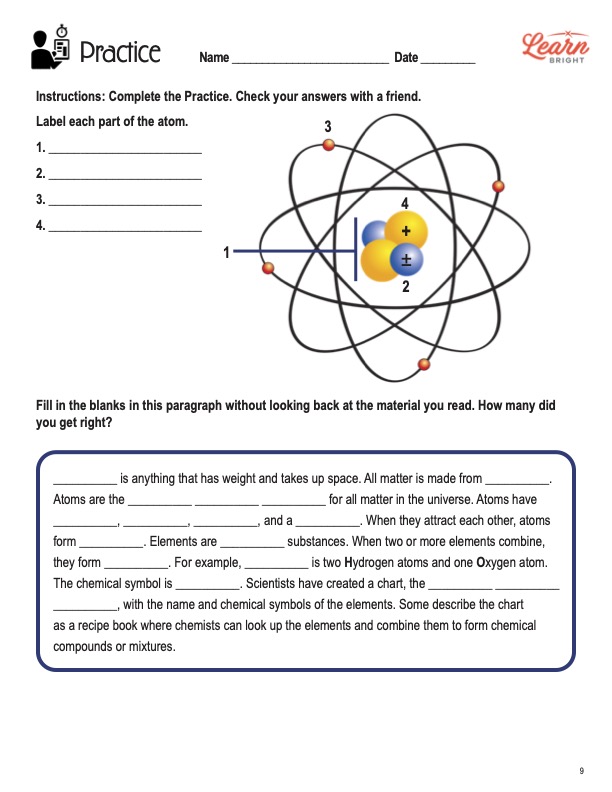
Metric System Handout/Worksheet Integrated Science 1 … The basic parts of an atom are protons, neutrons and electrons. Electrons were discovered first by JJ Thomson in 1897. He found that electrons are tiny, negatively charged particles. Next, protons were discovered by Ernst Rutherford in 1911. Protons are small, positively charged particles that reside in the nucleus.
Protons neutrons and electrons practice worksheet
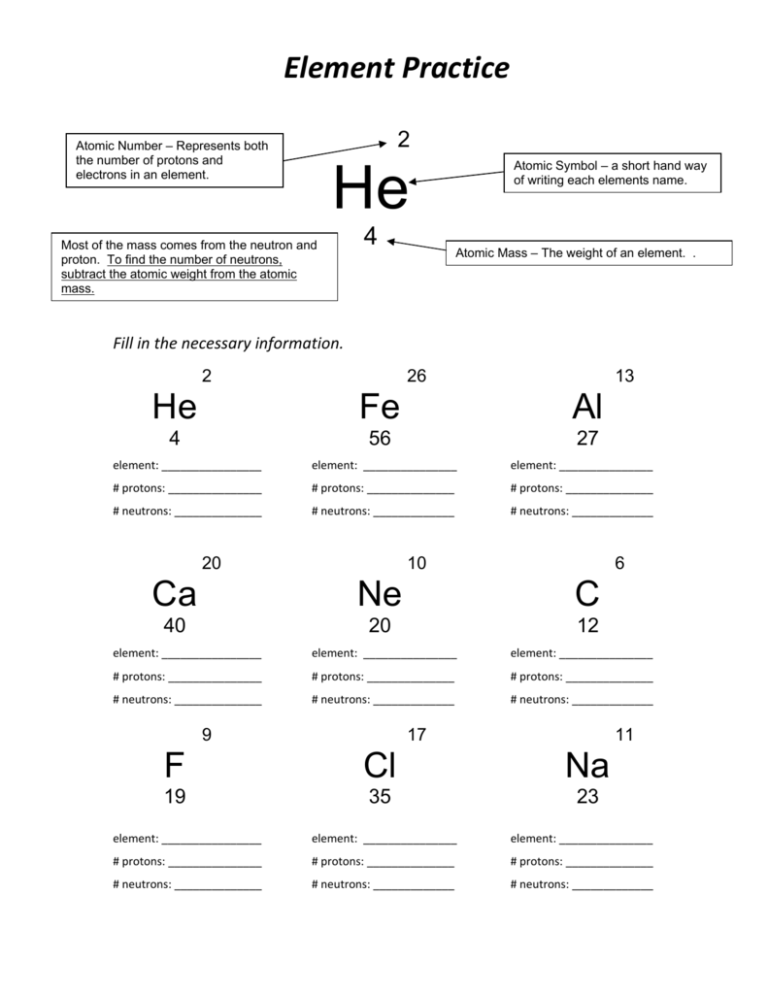
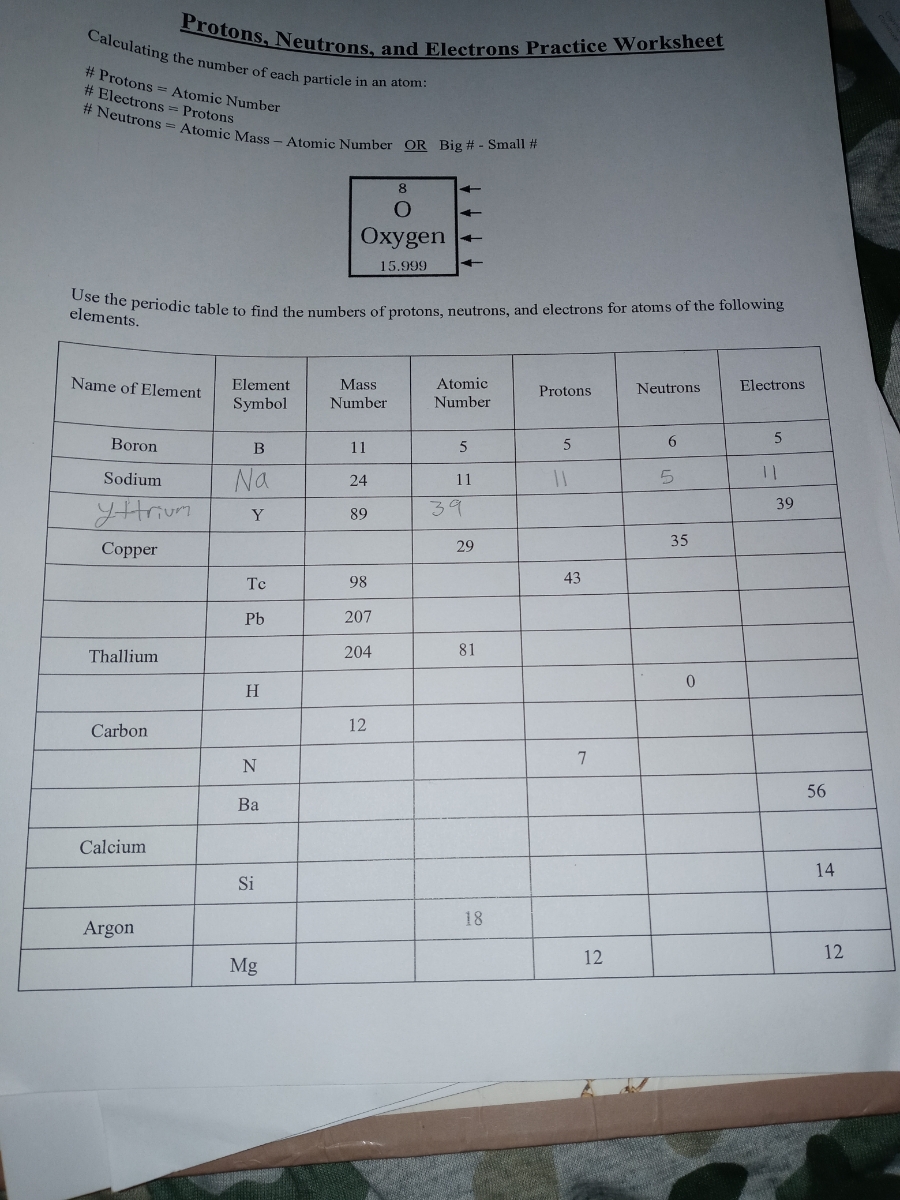
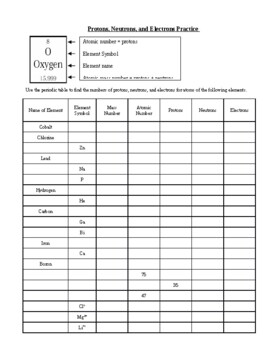
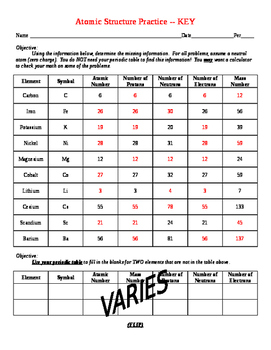
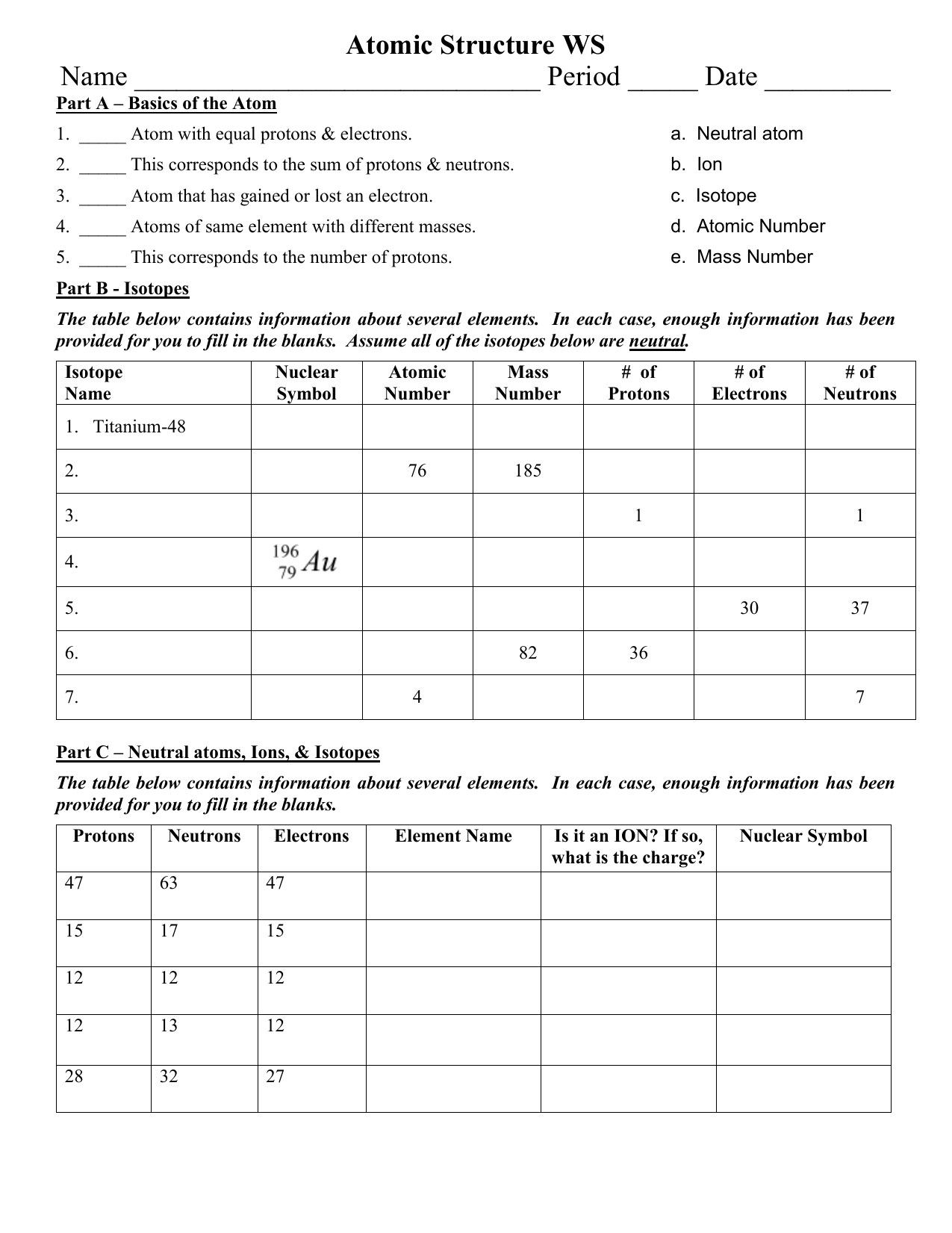
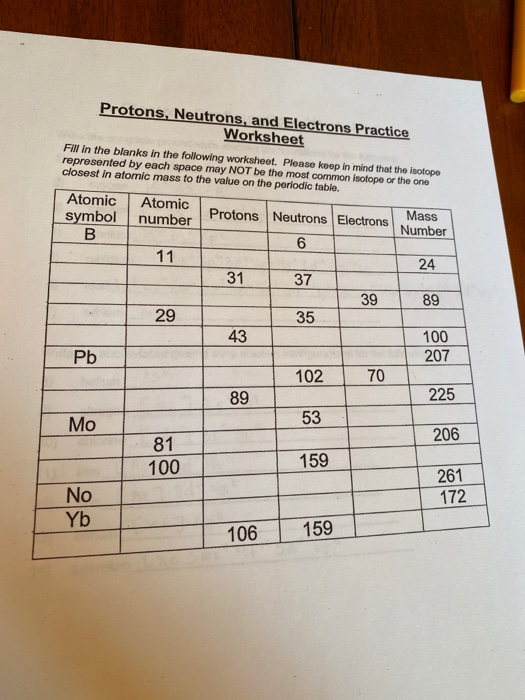
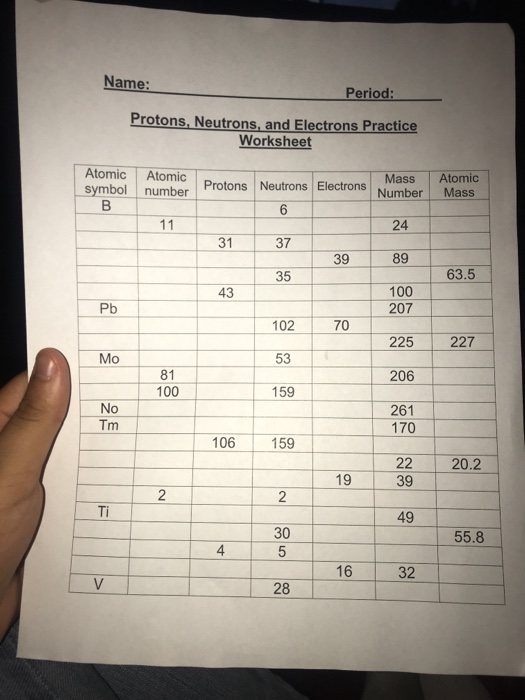
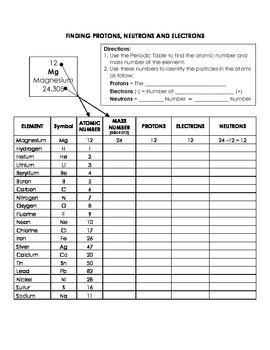
Protons, Neutrons, and Electrons Practice Worksheet - SMATCOE Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass – Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 Cambridge IGCSE Chemistry Coursebook 4th Edition CD-ROM 11 Petrochemicals and polymers 275 Study and revision skills CD1 11.1 Petroleum 276 Self-assessment practice tests CD19 11.2 Alternative fuels and energy sources 282 Practice exam-style papers and marking schemes CD66 11.3 Addition polymerisation 284 CD131 Syllabus contents table 11.4 Condensation polymerisation 287 CD148 Syllabus coverage by book …
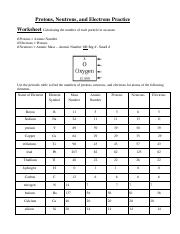
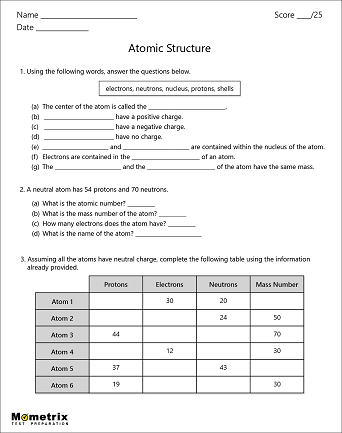
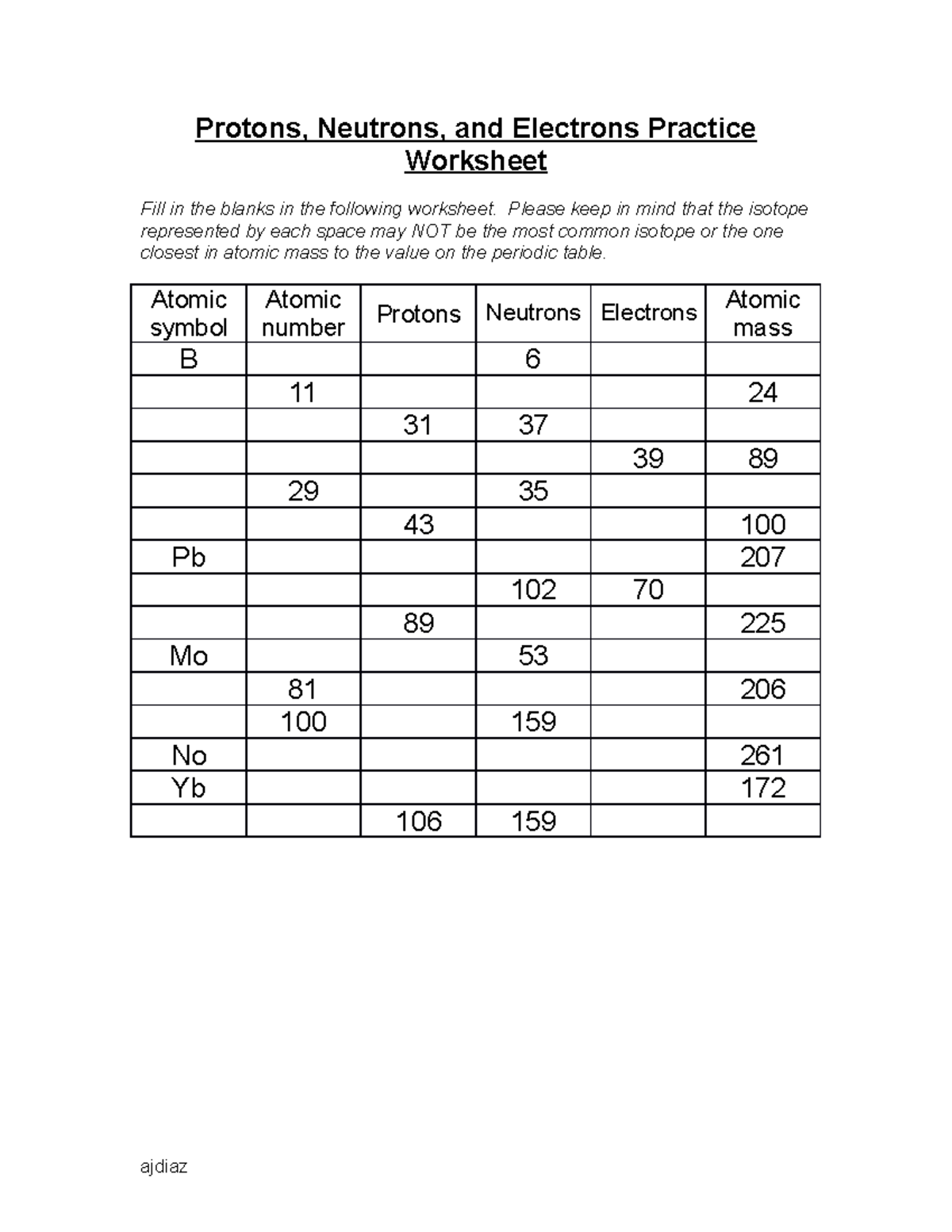
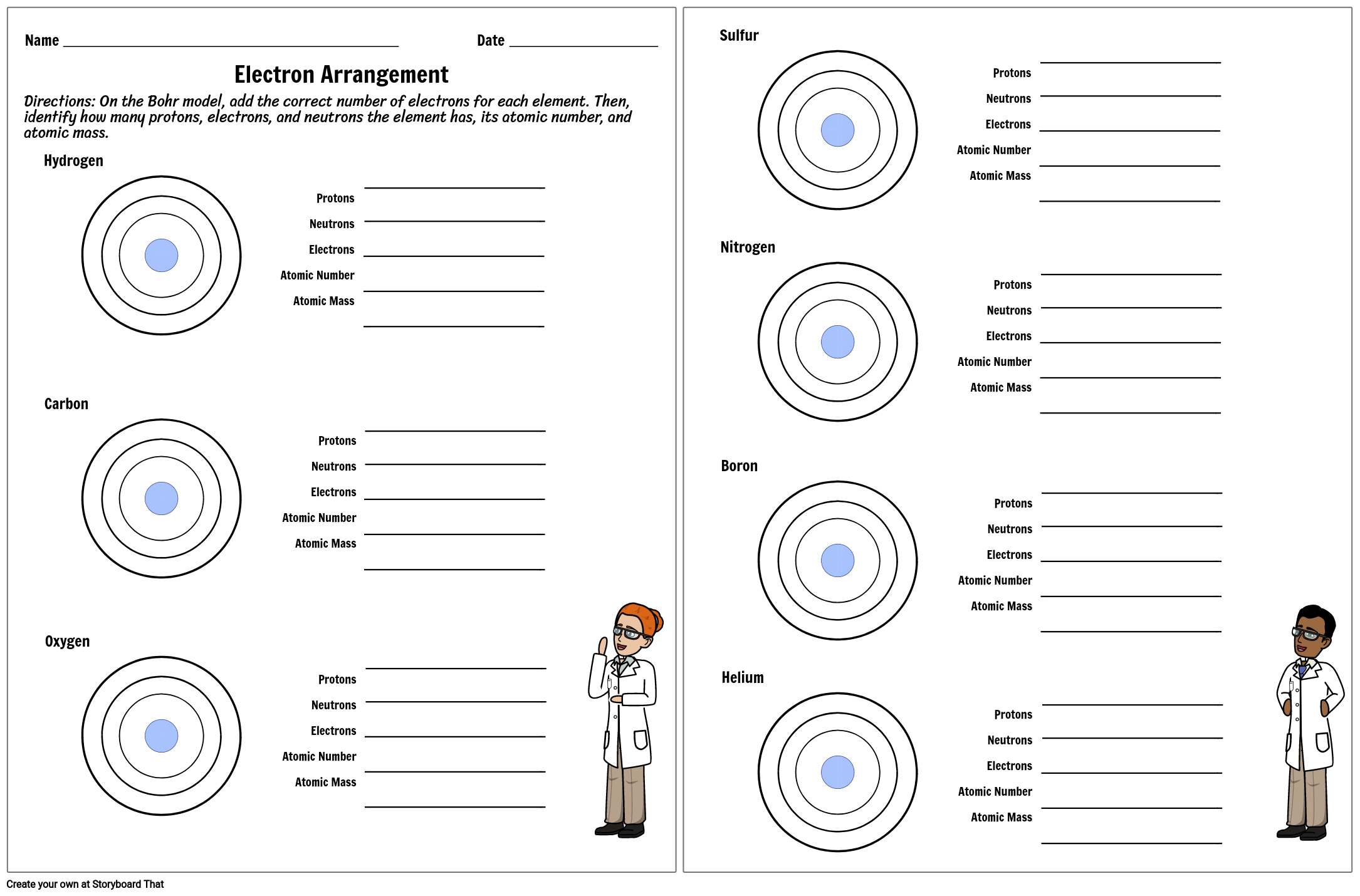
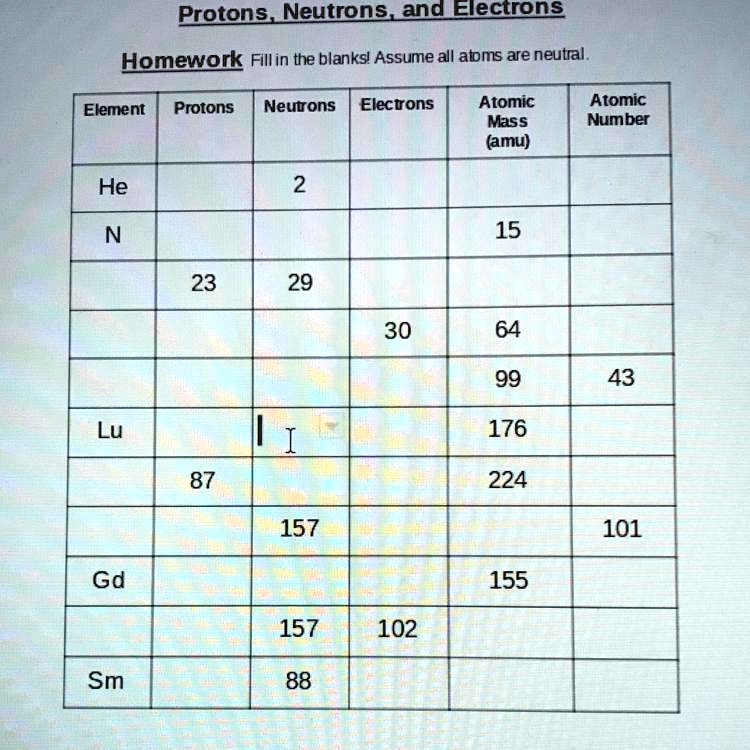
Protons neutrons and electrons practice worksheet. Protons, Neutrons, and Electrons Practice Worksheet Protons, Neutrons, and Electrons Practice Worksheet Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass B6 11 24 31 ... Atomic Structure Worksheets Click the buttons to print each worksheet and associated answer key. Atoms and Molecules. ... Practice Sheet 2. ... The data given may include element name, symbol, atomic number, number of sub atomic-particles (protons, electrons, neutrons), and any charge that may exist. Electron Configuration. Show us where the electrons are located. Isotopes Worksheet - Perth Amboy Public Schools Iodine-88 # of protons 53 53 # of neutrons 32 35 # of electrons 53 53 Germanium-65 Germanium-68 # of protons 32 32 # of neutrons 33 36 # of electrons 32 32 Carbon -10 Carbon -12 # of protons 6 6 # of neutrons 4 6 # of electrons 6 6 Chromium -54 Chromium -56 # of protons 24 24 # of neutrons 30 32 # of electrons 24 24 Titanium -22 Titanium -25 ... How to Calculate Total Charge in Coulombs of an Arrangement of Protons … Protons:Protons are subatomic particles that, together with neutrons, form the nucleus of an atom. Protons are larger and more massive than electrons, and carry a positive charge, {eq}1.6\times 10 ...
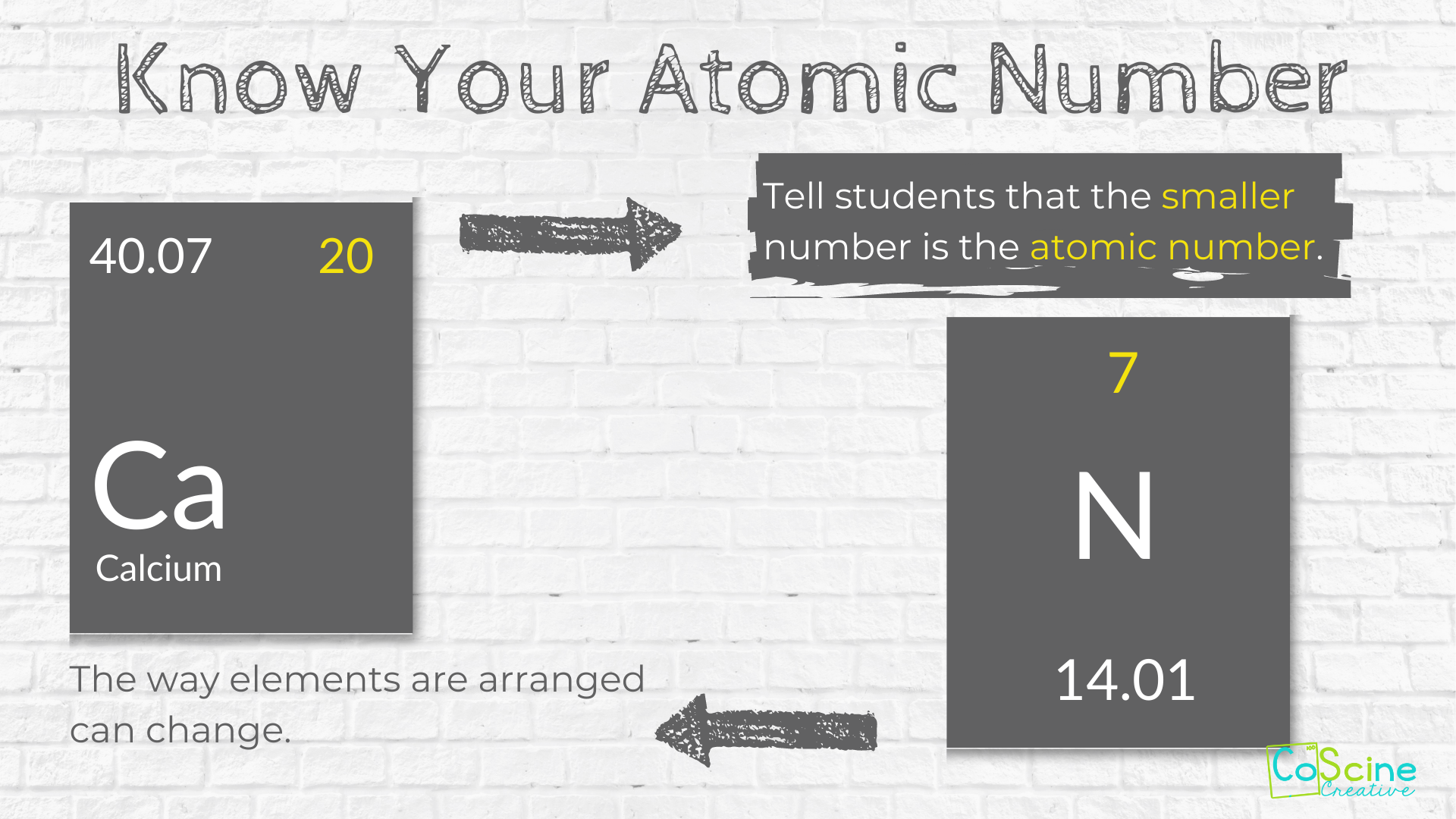
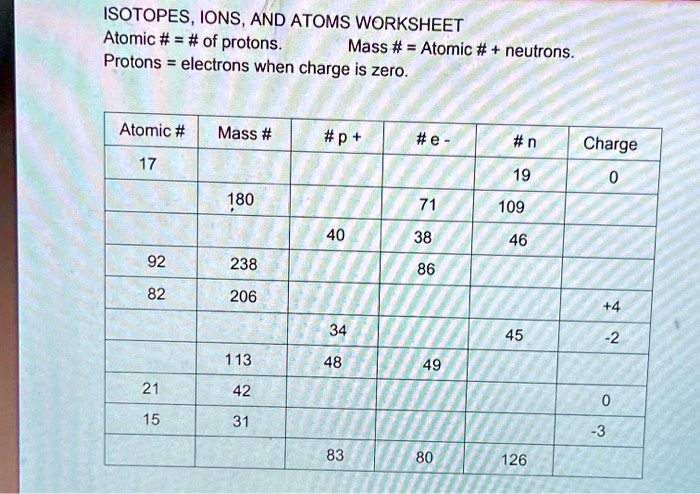
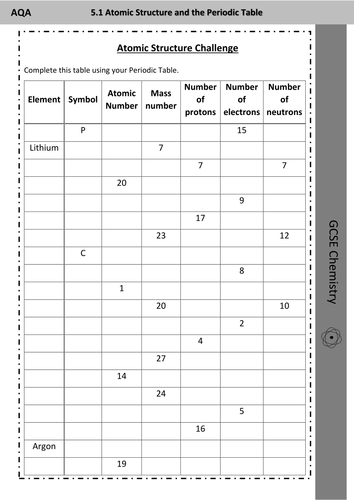
Atomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus.For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements.In an ordinary uncharged atom, the … What is an Atom -Basics for Kids - YouTube Visit for more free science videos for kids.What is an Atom? A good video explaining atomic structure & molecules formation. An a... Cambridge IGCSE Chemistry Coursebook 4th Edition CD-ROM 11 Petrochemicals and polymers 275 Study and revision skills CD1 11.1 Petroleum 276 Self-assessment practice tests CD19 11.2 Alternative fuels and energy sources 282 Practice exam-style papers and marking schemes CD66 11.3 Addition polymerisation 284 CD131 Syllabus contents table 11.4 Condensation polymerisation 287 CD148 Syllabus coverage by book … Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79
Protons, Neutrons, and Electrons Practice Worksheet - SMATCOE Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass – Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element


![Protons Neutrons And Electrons Practice Ws [6nq85590eznw]](https://idoc.pub/img/crop/300x300/6nq85590eznw.jpg)















0 Response to "44 protons neutrons and electrons practice worksheet"
Post a Comment