39 calorimetry practice worksheet answers
Calorimetry Practice Answer Key Calorimetry Practice Problems (Answers) 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oC? 3135J 3140J (rounded answer for sig. figs.) 2. How many grams of water can be heated from 20.0 oC to 75oC using 12500.0 Joules? 119.6 g 120 g (rounded answer for sig. figs) 3. Calorimetry Practice Problems Answer Sheet - myilibrary.org Calorimetry Worksheet 1) If 0.315 moles of hexane (C 6 H 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion of hexane if the water temperature rises 55.4 °C? The specific heat capacity of water is 4.184 J/g °C. H = ms T H = (5,650 grams H 2 O) (4.184 J/g °C) (55.4 °C) H = 1310 kJ
Calorimetry Problems 1 Worksheet Answer Key Calorimetry Practice Worksheet. 1). Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this ... Specific Heat Worksheet KEY. Specific Heat Worksheet. Name (in ink):. C = q/mAT, where q = heat energy, ...

Calorimetry practice worksheet answers
Calorimetry Problems Worksheet Answers - qstion.co Calorimetry practice problems answers 1. B) the heat given off by the combustion of the fat measured in kj/g 2. Cal of energy is lost from a 125 g object the. The temperature of the water decreases to 17.0oc. A) the heat absorbed by the water; Find the change in heat content of the system. PDF Calorimetry Practice Worksheet (Section 17.2) - NOTAPROTON Calorimetry Practice Worksheet (Section 17.2) 1.) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this compound causes the temperature of the water to rise 45.00C, what is the molar heat of combustion of compound A (in kJ/mole)? The heat capacity of water is 4.18 J/g 0C. ∆H = -q Practice Questions with Answers & Explanations - BYJUS Answer: absorbed Explanation: Inside a closed system, the heat energy lost by a hot object is equivalent to the total heat energy absorbed by the cold body. 9) The concept of calorimetry is built around the law of _____. Answer: conservation energy. Explanation: The concept of calorimetry is built around the law of conservation energy.
Calorimetry practice worksheet answers. Calorimetry Practice Worksheet Answer Key - myilibrary.org Calorimetry problems worksheet answer key (QSTION.CO) - Calorimetry worksheet 1 if 0315 moles of hexane c 6 h 14 is combusted in a bomb calorimeter containing 565 liters of water calculate the molar heat of combustion of hexane if the water temperature rises 554 c. We have seen in previous chapters that energy is one of the fundamental concepts ... Calorimetry Practice Problem Worksheets - K12 Workbook Worksheets are Calorimetry work w 337, Ii calorimetry work, Chemistry work heat and calorimetry problems answers, Calorimetry practice problems answers, Thermochemistry work 1, Calorimeter practice with answers, Calorimetry practice problems with answers, Calorimetry problems with answers. *Click on Open button to open and print to worksheet. 1. ️Calorimetry Worksheet Answers Free Download| Qstion.co Calorimetry worksheet answers (QSTION.CO) - 3135j 3140j rounded answer for sig. Calorimetry worksheet 1 if 0315 moles of hexane c 6 h 14 is combusted in a bomb calorimeter containing 565 liters of water calculate the molar heat of combustion of hexane if the water temperature rises 554 c. 2018 week 1 online lab. We have seen in previous chapters that energy is one of the fundamental concepts ... Calorimetry Practice Worksheet - Calorimetry Practice... - Course Hero Calorimetry Practice Worksheet 1) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this compound causes the temperature of the water to rise 45.0 0 C, what is the molar heat of combustion of compound A? The heat capacity of water is 4.184 J / g 0 C. 2) Compound B is burned in a bomb calorimeter that contains 1.50 liters of water.
PDF Calorimetry Worksheet - Laney College Calorimetry Worksheet 1) If 0.315 moles of hexane (C 6 H 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion of hexane if the water temperature rises 55.4 °C? The specific heat capacity of water is 4.184 J/g °C. H = ms T H = (5,650 grams H 2 O) (4.184 J/g °C)(55.4 °C) H = 1310 kJ PDF Calorimetry Worksheet W 337 - Everett Community College Calorimetry Worksheet W 337 Everett Community College Tutoring Center Student Support Services Program C p (H 2 O) = 4.184 J / g 0C H = mC p T 1) A compound is burned in a bomb calorimeter that contains 3.00 L of water. If the combustion of 0.285 moles of this compound causes the temperature of the water to Calorimetry Problems Worksheet Answers Calorimetry Practice Problems | PDF | Calorie | Chemistry - Scribd Answer: 3.34 ×10 5 cal . 4. The heat capacity of aluminum is 0.900 J/goC. a. How much energy is needed to raise the temperature of 8.50 x 102 g ... Specific Heat Worksheet Show all work and proper units. calorimetry worksheet 1 answers 29 Calorimetry Practice Worksheet Answers - Free Worksheet Spreadsheet dotpound.blogspot.com. calorimetry. Calorimetry For AS Level | Teaching Resources . calorimetry. Calorimetry Worksheet Answer Key Best Of Calorimetry Lab 9th 10th Grade . 9th. Bomb calorimetry worksheet a bomb calorimeter is used to determine the.
Calorimetry Practice Worksheet17.2answers.pdf - Calorimetry... View Homework Help - Calorimetry Practice Worksheet17.2answers.pdf from ENGR 313 at Liberty University. Calorimetry Practice Worksheet (Section 17.2) 1.) Compound A is burned in a bomb calorimeter Calorimetry worksheet answers.docx - Heat capacity and... View Calorimetry worksheet answers.docx from CHEM 1212 at Kennesaw State University. Heat capacity and Calorimetry 1. How much heat is needed to warm 250 grams of water from 22⁰C to 98⁰C? Calorimetry Problems Worksheet Answer Key - myilibrary.org Honors Chemistry Worksheet: Heat Calculations Answer Key 1.) 107 kJ 2.) Cp= 1.98 J/g.°C ~ Acetic Acid 3.) 18600 J 4.) 5180 000 J = 1.8x10 J 5.) 268 g ... 14.) calorimetry equation; Cp= 0.10J/g.°C 15.) calorimetry equation; Cp= 0.42J/g.°C 16.) 18.0°C . Title CalorimetryAnswerKey Author ... PDF Calorimetry Practice Problems Calorimetry Practice Problems (Answers) 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oC? 3135J 3140J (rounded answer for sig. figs.) 2. How many grams of water can be heated from 20.0 oC to 75oC using 12500.0 Joules? 119.6 g 120 g (rounded answer for sig. figs) 3.
PDF Calorimetry Problems Worksheet - bremertonschools.org Calorimetry Problems Name_____ Per____Date_____ q sur = m x C x T q rxn = -q sur q = heat m = mass T = T f - T i C = specific heat (for water = 4.184 J/goC) 1. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oC when 207 J of heat is added? 2.
Heat Capacity Calorimetry Worksheet answers - StuDocu Explain. Low specific heat. For the same reasoning as question # 1 It takes less energy to raise 1 gram of a material's temperature with a low specific heat, where as 100 J of energy would have less of an effect on a material with a high heat capacity. A 5 g mass of a metal was heated to 100 oC and then plunged into 100 g of water at 24 oC.
️Calorimetry Worksheet 1 Answers Free Download| Qstion.co Calorimetry worksheet 1 answers. B) the heat given off by the combustion of the fat measured in kj/g 2. A calorimeter containing 1.50 l of water at 25.0° c is warmed to 72.0° c when 5.00 g of fat is burned. A) the heat absorbed by the water; Fill each grid space with a properly concise answer. The factor that links these two is heat capacity.
Practice Questions with Answers & Explanations - BYJUS Answer: absorbed Explanation: Inside a closed system, the heat energy lost by a hot object is equivalent to the total heat energy absorbed by the cold body. 9) The concept of calorimetry is built around the law of _____. Answer: conservation energy. Explanation: The concept of calorimetry is built around the law of conservation energy.
PDF Calorimetry Practice Worksheet (Section 17.2) - NOTAPROTON Calorimetry Practice Worksheet (Section 17.2) 1.) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this compound causes the temperature of the water to rise 45.00C, what is the molar heat of combustion of compound A (in kJ/mole)? The heat capacity of water is 4.18 J/g 0C. ∆H = -q
Calorimetry Problems Worksheet Answers - qstion.co Calorimetry practice problems answers 1. B) the heat given off by the combustion of the fat measured in kj/g 2. Cal of energy is lost from a 125 g object the. The temperature of the water decreases to 17.0oc. A) the heat absorbed by the water; Find the change in heat content of the system.

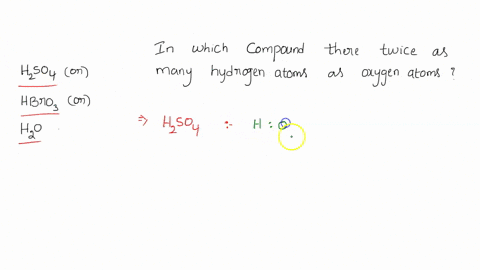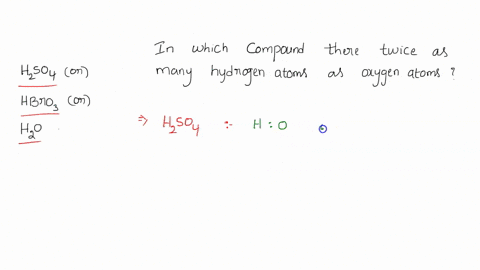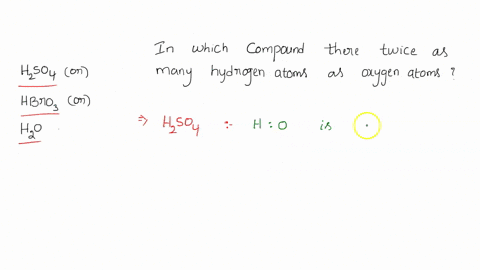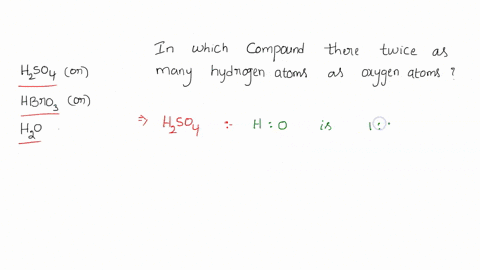
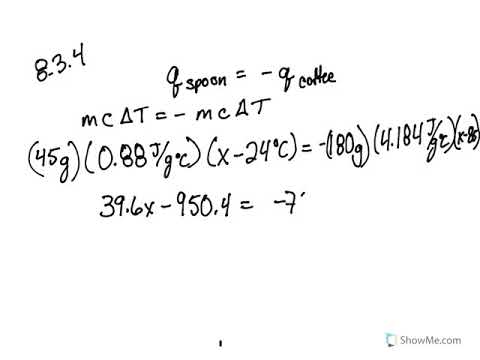
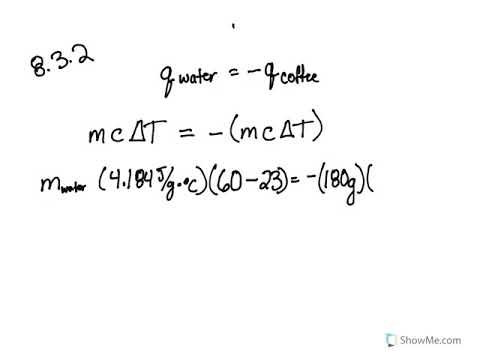








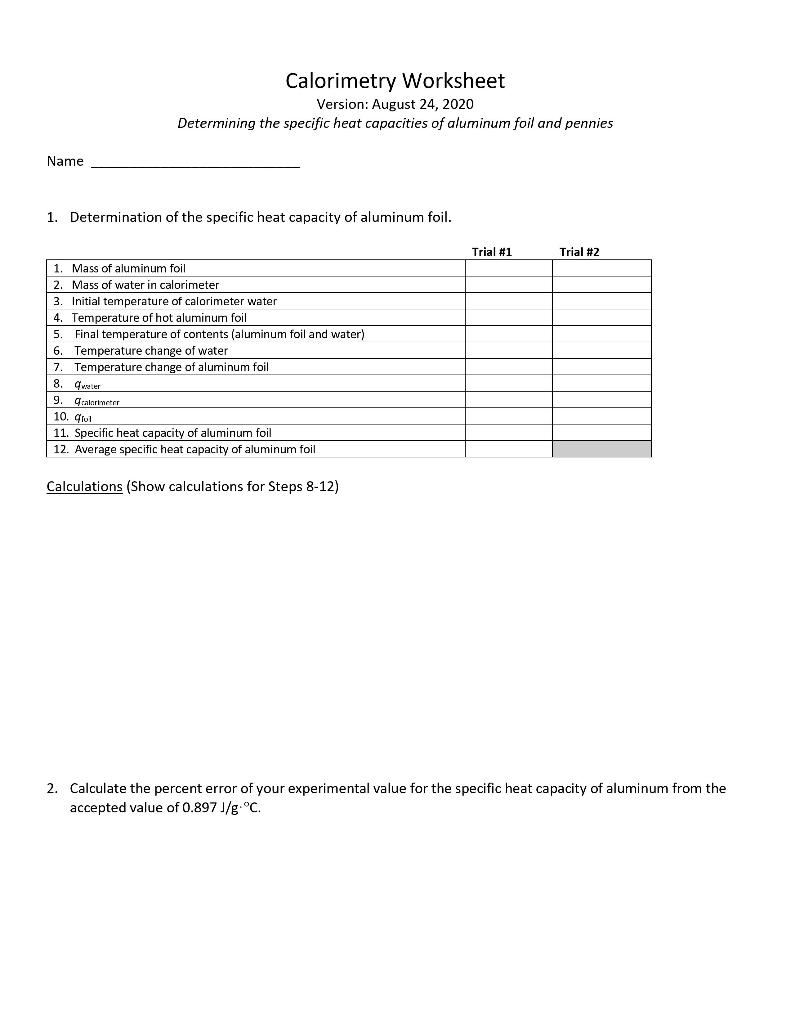


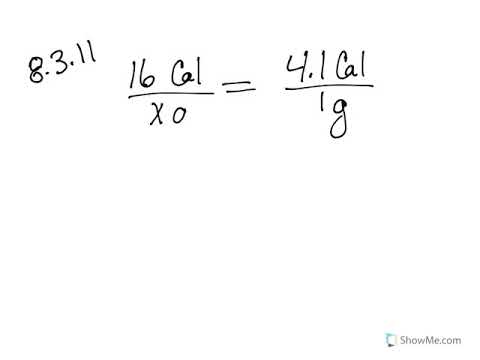











0 Response to "39 calorimetry practice worksheet answers"
Post a Comment