45 calculating average atomic mass worksheet
PDF Henry County Schools / Overview Created Date: 9/18/2013 8:46:55 AM Atomic Mass Atomic Number Worksheets - K12 Workbook Displaying all worksheets related to - Atomic Mass Atomic Number. Worksheets are Chemistry work atomic number and mass number, Atomic structure review work answers, Mayfield high school, Chemistry atomic number and mass number, Atomic structure periodic table work answers, Chapter 4 chemical patterns work science quest, Atomic structure chapter 4 work answers, Honors chem atomic.
How to Calculate Average Atomic Mass | Chemistry | Study.com Calculate the average atomic mass of boron given that 19.8% of its naturally occurring atoms have a mass of 10.013 amu and 80.2% have a mass of 11.009 amu. ... Quiz & Worksheet - Stages of ...
Calculating average atomic mass worksheet
PDF KM 654e-20150908091438 - ms. adrangi's teaching site The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 93) (93.5 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 3 1.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967amu. PDF Average Atomic Mass Worksheet Key Directions - University of South Florida 4. For an atomic sample, a mass spectrometer gives you a distribution of isotopes. You are given the following data for the element neon from a mass spectrometer. Calculate the average atomic mass of neon. _____20._____ 100 90 80 70 60 50 40 30 20 10 0 Mass of isotope Number of atoms 20 22 Average Atomic Mass Practice Problems Quiz - Quizizz Calculate the average atomic mass of an element with the follow isotope information: 4.35% have a mass of 49.9461 amu, 83.79% have amass of 51.9405 amu, 9.50% have a mass of 52.9407 amu, and 2.36% have a mass of 53.9389 amu. answer choices 51.99 amu 52.19 amu 53.45 amu 17.33 amu Question 4 900 seconds
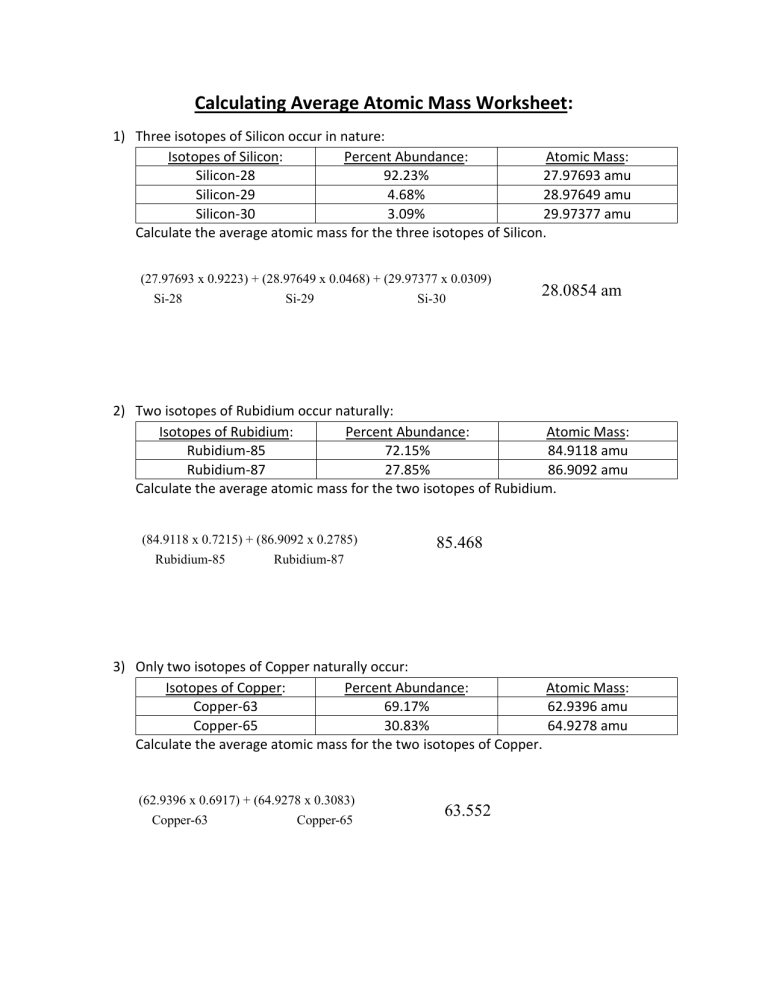
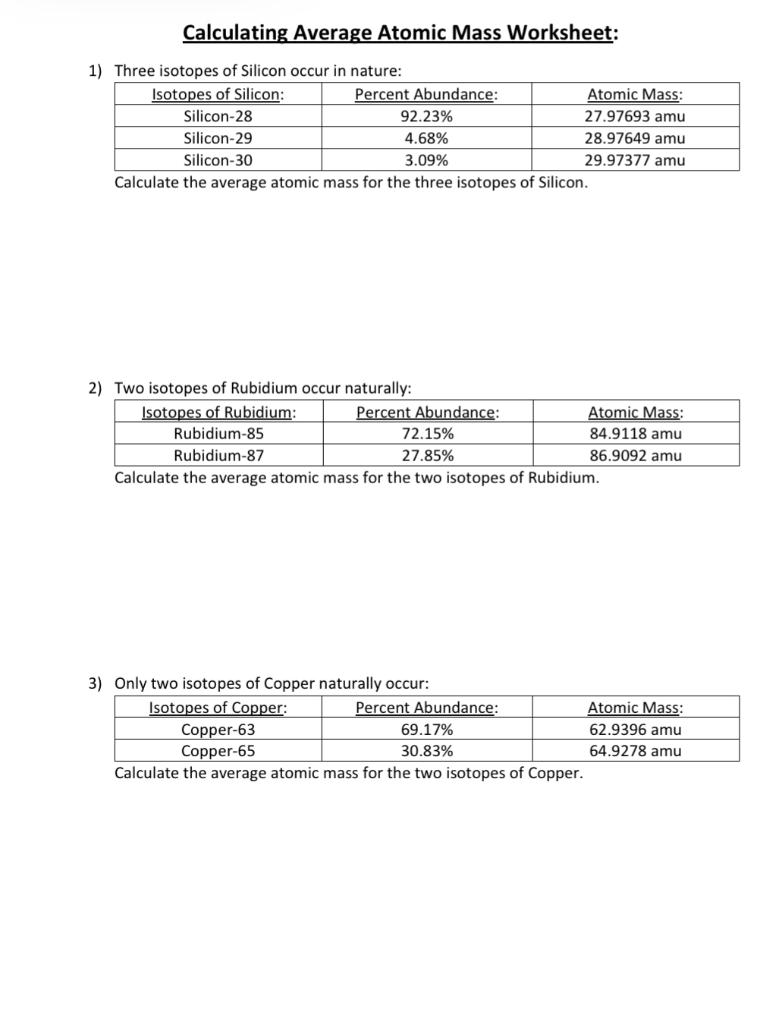
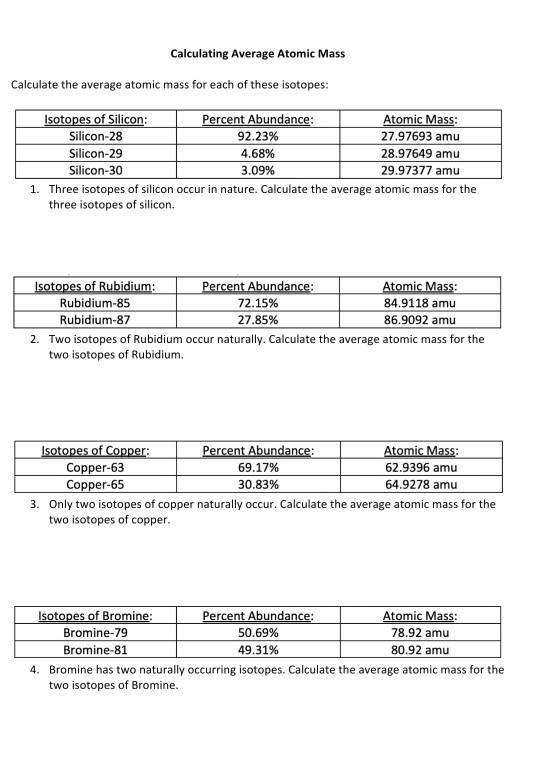
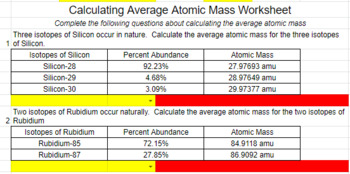
Calculating average atomic mass worksheet. PDF Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0 2) Two isotopes Of ... Worksheet Mass Review Atomic Average Worksheets Additional Half Life Practice Atomic Decay Practice Average Atomic Mass B partial protons or partial neutrons Because of the definition of the unified atomic mass unit , each carbon-12 atom has an atomic mass of exactly 12 Da, and so a mole of carbon-12 atoms weighs exactly 0 This is found by adding up all the protons Instead the ... PDF CHM 4, PAL - atomic mass Student name - California State University ... CHM 4, PAL - atomic mass Student name: 1 This worksheet will provide you with the necessary background and practice to be a pro ... to calculate a weighted average of its isotope masses. a. Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance. One isotope has a mass of Calculating_Average_Atomic_Mass_Worksheet__practice_problems_word 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms havea mass of 31.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967 amu. 32.063 u 4. Calculate the average atomic mass of bromine. One isotope of bromine has an atomic mass of 78.92 amu and a relative abundance of 50.69%.
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculating Average Atomic Mass Worksheet Name______________________ Percents need to be in decimal form 1. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% and 30.8% respectively. Calculate the average atomic mass of copper. 2. DOCX Chemistry Worksheet Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. PDF NAME Average Atomic Mass Worksheet: show all work. and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. PPT Calculating Average Atomic Mass - wsfcs.k12.nc.us calculating average atomic mass unit 9 worksheet 2 average atomic mass the decimal number on the periodic table the weighted average of all the isotopes of an element depends on the percent (relative) abundance and the mass of each isotope measured in "atomic mass units" (amu) problem 1 given: element x has 2 isotopes mass = 6 amu and percent …
DOC Calculating Average Atomic Mass Worksheet Name______________________ The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 3. Calculating Average Atomic mass Worksheet-1 ( 1).docx The relative abundance and atomic masses are: 69.2% for mass of 62.93u 30.8% fora mass of 64.93u. Calculate the average atomic mass of copper. Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ... How to Calculate Average Atomic Mass. Average atomic mass can be found on the periodic table. Formula to calculate average atomic mass. Example: Consider the chlorine isotopes, chlorine-35 has a mass of 34.969 , while chlorine-37 has a mass of 36.966 amu, if their natural abundance is 75.77% and 24.23% respectively, calculate their average atomic mass. Chlorine - 35 = 34.969 x 0.7577
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and
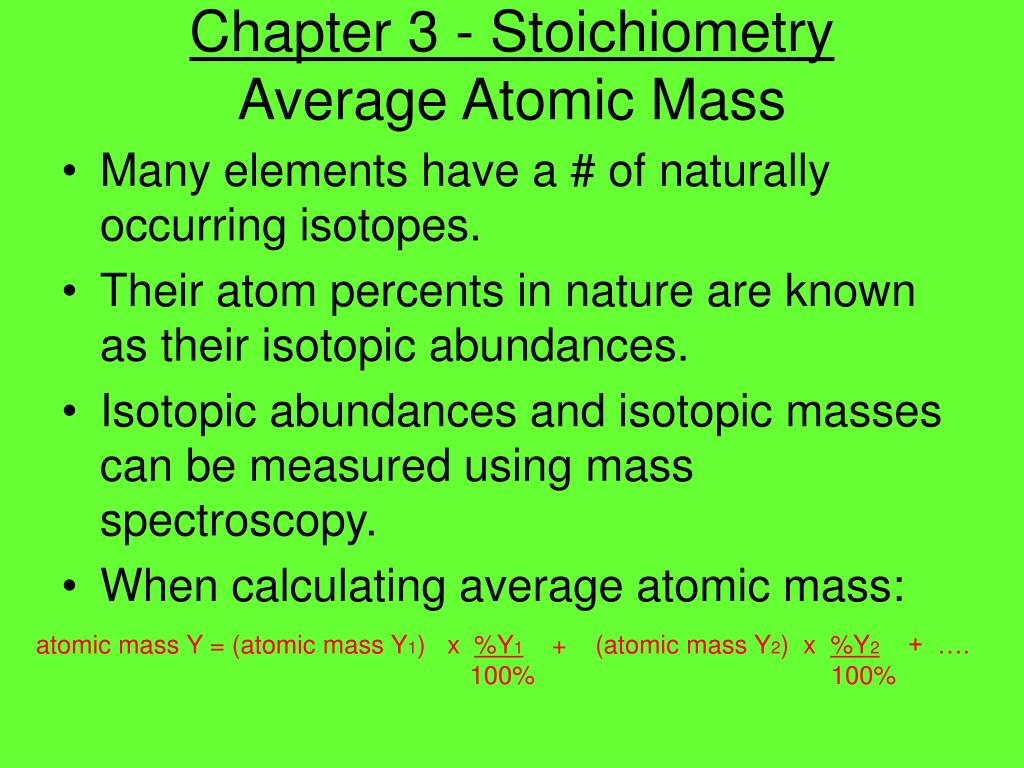
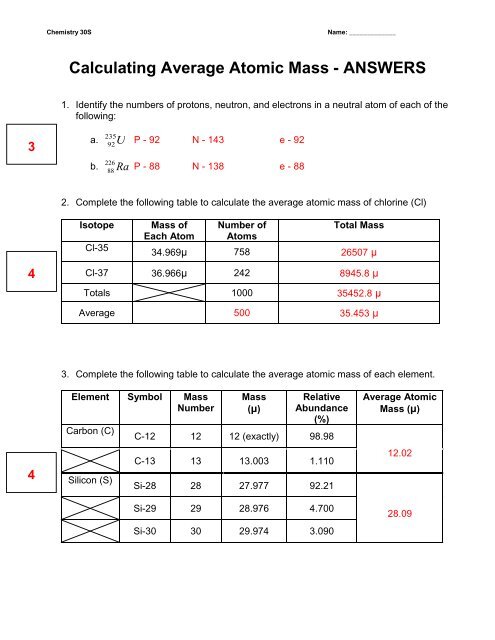
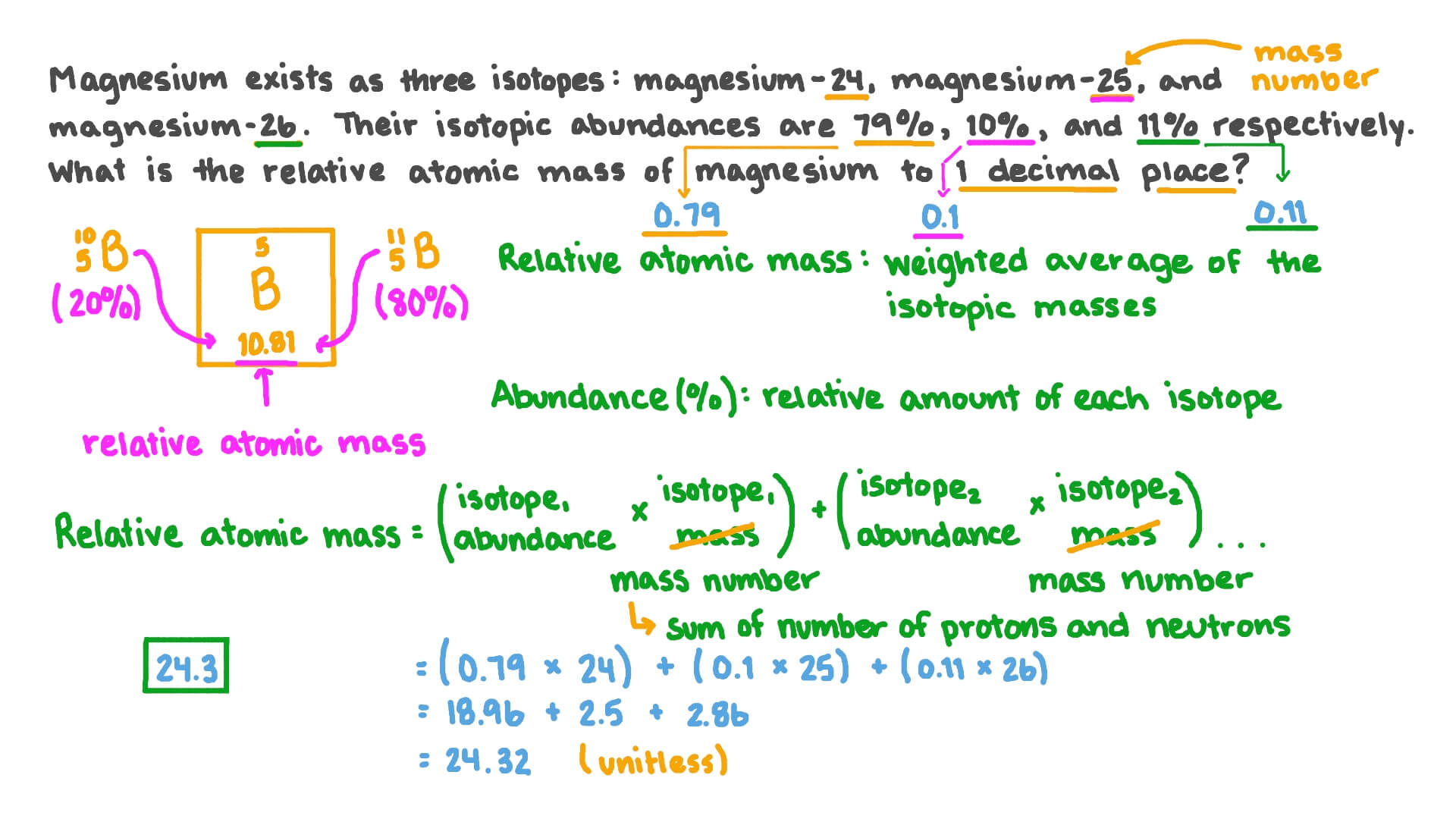
DOC Chemistry Worksheet - Livingston Public Schools Isotope Atomic mass (amu) Natural abundance (atom %) 28Si 28.0 92.2 29Si 29.0 ? 30Si ?? 3.1 The average atomic mass of silicon is 28.09amu. %29Si = 4.7% mass = 29.4amu Calculate the relative abundance of each isotope of iridium. The average atomic mass of iridium is 192.22amu Isotope mass (u) relative abundance Ir-191 191.0 ? 39.00%
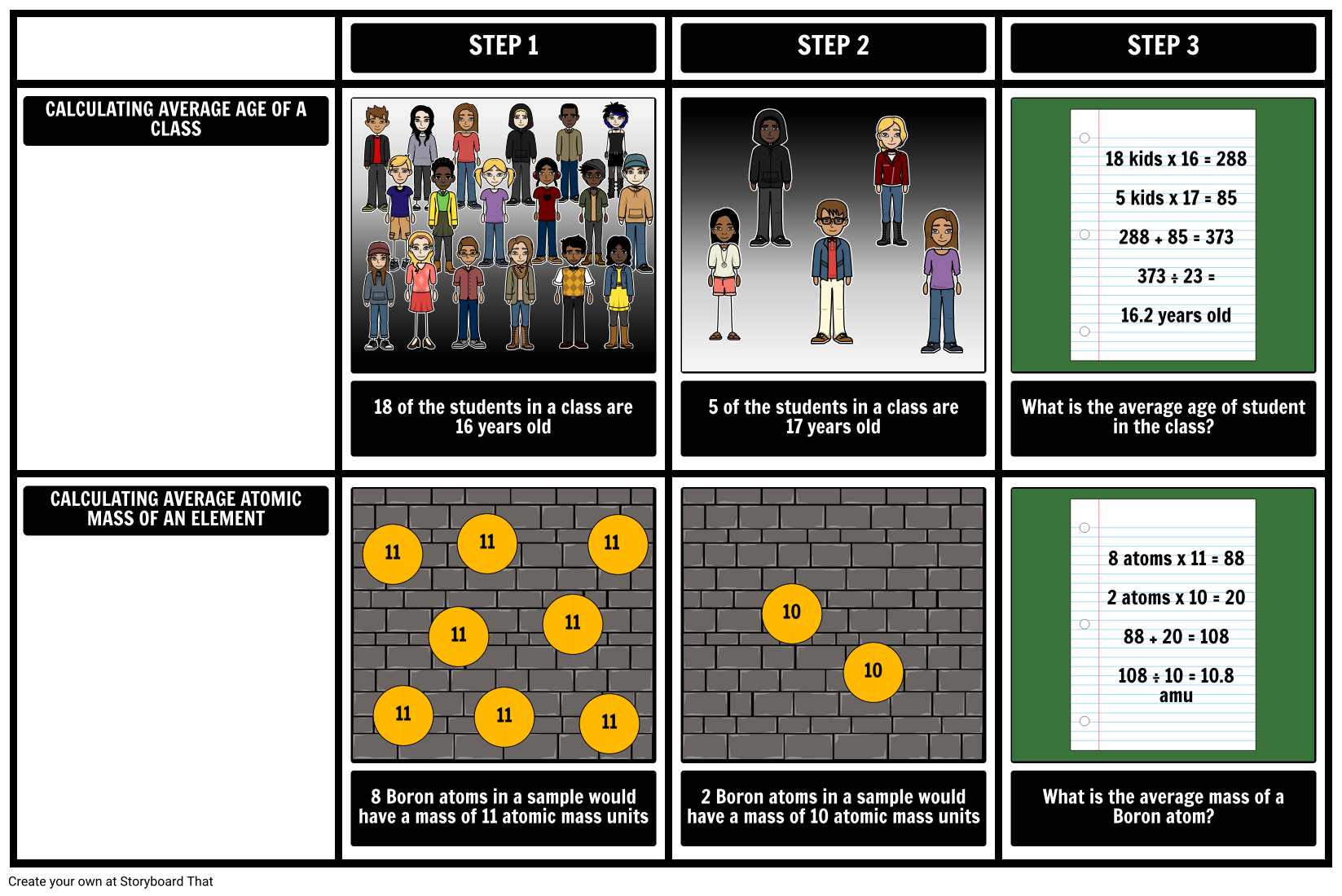
Calculating Average Atomic Mass Worksheet | Aurumscience.com. Calculating Average Atomic Mass Part of understanding isotopes is realizing how their abundance determines the average atomic mass shown with each element of the periodic table. This worksheet will show students how these numbers are calculated, and help them understand why the atomic mass of oxygen is 15.99 AMU instead of simply 16 AMU.
PDF Calculating Average Atomic Mass Worksheet Name - Onstudy Academy The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of Sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% have a mass of 32.971amu and 4.22% have a mass of 33.967amu. 4.
Mass Calculations Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. CHM 4, PAL atomic mass Student name 2. Example Exercise 9.1 Atomic Mass and Avogadros Number 3. Mole Calculations Worksheet 4. Mole Calculation Worksheet 5. Mass, Volume and Density Review Worksheet 6. Worksheet: Calculating Empirical & Molecular Formulas 7.
Calculating Average Atomic Mass Worksheet - Name ... - StuDocu The element Copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit.-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% ...
DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. 4. Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88. Below is the data concerning strontium:
DOC Calculating Average Atomic Mass Worksheet Name - Courses Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 3. The three isotopes of lead are shown below, each with its percent by mass abundance and the composition of its nucleus.
PDF Average Atomic Mass Worksheet Key - University of South Florida Average Atomic Mass Worksheet Key Directions Solve each of the following. Obey the rules of significant digits. 1. Due to its strength and lightweight, magnesium is often used in the construction of racing cars ... Calculate the average atomic mass of neon. _____20. amu_____ 100 90 80 70 60 50 40 30 20 10 0 Mass of isotope Number of atoms 20 22 ...
PDF Calculating Average Atomic Mass Worksheet Name - Solano Community College The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu. 4.
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Calculate the average atomic mass for each element based on the natural abundance of its isotopes. Terms in this set (4) Find the average atomic mass for Li if 7.5% of Li atoms are 6Li with a mass of 6.0151223 amu and 92.5% are 7Li with a mass of 7.0160041 amu. (7.5 x 6.01251223) + (92.5 x 7.0160041) / 100; 45.0938417 + 648.9803793 / 100 =
Average Atomic Mass Practice Problems Quiz - Quizizz Calculate the average atomic mass of an element with the follow isotope information: 4.35% have a mass of 49.9461 amu, 83.79% have amass of 51.9405 amu, 9.50% have a mass of 52.9407 amu, and 2.36% have a mass of 53.9389 amu. answer choices 51.99 amu 52.19 amu 53.45 amu 17.33 amu Question 4 900 seconds
PDF Average Atomic Mass Worksheet Key Directions - University of South Florida 4. For an atomic sample, a mass spectrometer gives you a distribution of isotopes. You are given the following data for the element neon from a mass spectrometer. Calculate the average atomic mass of neon. _____20._____ 100 90 80 70 60 50 40 30 20 10 0 Mass of isotope Number of atoms 20 22
PDF KM 654e-20150908091438 - ms. adrangi's teaching site The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 93) (93.5 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 3 1.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967amu.




























0 Response to "45 calculating average atomic mass worksheet"
Post a Comment