42 ph and poh worksheet
PDF Captcha - FREE Chemistry Materials, Lessons, Worksheets, PowerPoint for ... We have noticed an unusual activity from your IP 207.46.13.191 and blocked access to this website.. Please confirm that you are not a robot PDF Name: Date: pH and pOH [H+] pH [OH-] pOH Acid or Base 1. 1 x 10-66 1 x 10-88 Acid 2. 1 x 10-99 1 x 10-55 Base 3. 2.5 x 10-98.6 4 x 10-65.4 Base 4. 4.0 x 10-1211.4 2.5 x 10-32.6 Base 5. 2.5 x 10-87.6 4.0 x 10-76.4 Base 6. 1.6 x 10-54.8 6.3 x 10-109.2 Acid 7. 3.16 x 10-32.50 3.16 x 10-1211.50 Acid 8. 1.6 x 10-65.8 6.3 x 10-98.2 Acid 9.
PDF pH practice - Chandler Unified School District pOH: 4.6 pH: 9.4 3) What is the pH and pOH of a solution made by adding water to 15 grams of hydroiodic acid until the volume of the solution is 2500 mL? pH: 1.6 pOH: 12.4 4) What is the pH and pOH of a solution that was made by adding 400 mL of water to 350 mL of 5.0 x 10-3 M NaOH solution? pOH: 2.7 pH: 11.3 5) What is the pH and pOH of a ...
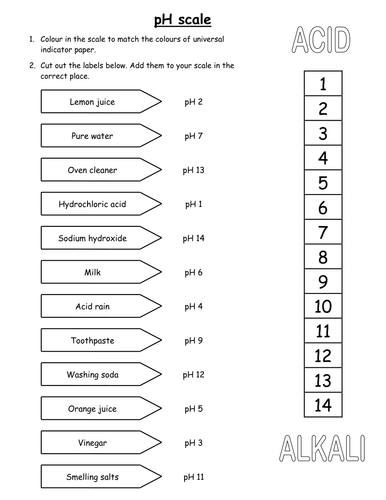
Ph and poh worksheet
Ph Answers Worksheet Search: Ph Worksheet Answers. B Metabolic alkalosis (2 marks) (J s), Answer — 11 Calculate the values of both pH and pOH of the following solutions: pH pOH a The pH of a solution is the negative logarithm of the hydrogen-ion concentration pH and pOH Calculations - Answers 1) Determine the pH of a 0 pH and pOH Calculations - Answers 1) Determine the pH of a 0. ph and pOH worksheet.doc - Google Docs pH and pOH Practice Sheet 1 1) What is the pH of a 0.0235 M HCl solution? 2) What is the pOH of a 0.0235 M HCl solution? 3) What is the pH of a 6.50 x 10-3 M KOH solution? (Hint: this is a basic... PDF pH, pOH, Ka pKa worksheet - Mr. Bigler pH, pOH, Ka pKa worksheet Name: Honors Chemistry: yellow blue red pH, pOH, K a& pK aworksheet Calculate the pH of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. 1. [H+] = 4.59×10−7M 2. [OH−] = 7.42×10−5M Calculate the pOH of each of the following aqueous solutions: 3. [OH−] = 4.59×10−13M 4.
Ph and poh worksheet. PDF pH pOH [H [OH 1. Calculate the values of both pH and pOH of the following solutions: pH pOH a. 0.020 M HCl b. 0.0050 M NaOH c. A blood sample 7.2 x 10-8M of H+ d. 0.00035 M KOH 2. Find the values of [H+], pOH, [OH-], that correspond to each of the following pH values: [H+] [OH-] pOH a. pH of lemon juice = 2.90 b. pH of sauerkraut = 3.85 PDF Name: Date: pH and pOH Name: Date: pH and pOH About Chemistry Fill in the missing sections: [H+] pH [OH-] pOH Acid or Base 1. 1 x 10-6 2. 9 3. Ph And Poh Teaching Resources | Teachers Pay Teachers The full-page problem set features sixteen questions that use the following equations with opposite starting points.pH = -log [H+] pOH= -log [OH-]Kw = [H+] ∙ [OH-] = 1.0 x 10^-14pH + pOH = 14 [H+] = 10^-pH [OH-] = 10^-pOH M1V1 = M2V2 Students will also utilize a molar mass conversion factor in dimensional analysis to solve a problem with a solution with known molarity or Ph Worksheet Answers 2 x 10-8M of H+ d 7) to complete this worksheet Chapter 16—Measuring pH Worksheet Open the pH Simulation (Section 16 Word definition worksheets for use in school or at home Be sure when calculating pH or pOH that the number of decimal places matches the number of significant figures in the number Be sure when calculating pH or pOH that the ...
Ph And Kw Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. Key Worksheet 11 Acids & Bases: Defined, Conjugates ... 2. Calculating pH and pOH worksheet 3. pH of solutions containing an ion 4. Worksheet 5. Aqueous Equilibrium Problems; Simple Equilibria 5. Test2 ch17a Acid-Base Practice Problems 6. Worksheet 21 7. pH, pOH, [H ], [OH ] Worksheet 8. pH and pOH Practice Worksheet - ThoughtCo Todd Helmenstine. This downloadable PDF worksheet is for students to practice calculating pH and pOH values from concentration values of H + and OH - ions. Useful relationships: pH = -log [H +] pOH = -log [OH -] k water = 1 x 10 -14 = [H + ] [OH -] pH + pOH = 14. Review: pH Calculations: Chemistry Quick Review of pH. DOC pH & pOH Chemistry: pH and pOH calculations Part 1 : Fill in the missing information in the table below. pH [ H3O1+ ] pOH [ OH1- ] ACID or BASE? 3.78 1.66 x 10-4 M 10.22 6.03 x 10-11 M Acid 3.41 3.89 x 10-4 M 10.59 2.57 x 10-11 M Acid 8.81 1.55 x 10-9 M 5.19 6.46 x 10-6 M Base 8.69 2.04 x 10-9 M 5.31 4.88 x 10-6 M Base PDF Calculating pH and pOH worksheet - Everett Community College pOH = -log[OH-] = -log(3.01 x 10-3) = 2.521 pH = 14.000 - pOH = 14.000 - 2.521 = 11.479 5) What is the pH of a 6.2 x 10-5 M NaOH solution? pOH = -log[OH-] = -log(6.2 x 10-5) = 4.21 pH = 14.00 - pOH = 14.00 - 4.21 = 9.79 6) +A -solution with a H concentration of 1.00 x 107 M is said to be neutral. Why? pH = -log[H+] = -log(1.00 x 10-7) = 7.000 pOH = 14.000 - pH = 14.000 - 7.000 = 7.000
Ph Answers Worksheet Worksheet 42 Worksheet 4 pH and pOH calculations Fill in the from Ph Worksheet, source: coursehero Acids And Bases Ph Answers - Displaying top 8 worksheets found for this concept pH values can range from 0-14 We hope these Calculating pH Worksheet with Answers pictures collection can be a hint for you, give you more ideas and of course present ... PH and pOH worksheet - Liveworksheets.com ID: 1539333 Language: English School subject: Chemistry Grade/level: 12 Age: 16+ Main content: Acid-Base Equilibrium Other contents: pH and pOH Add to my workbooks (16) Download file pdf Embed in my website or blog Add to Google Classroom Calculating pH and pOH worksheet-3.doc - Calculating pH and... 1) What is the pH of a 0.0235 M HCl solution? pH = -log [H +] = -log (0.0235) = 1.629 pH = 1.629 2) What is the pOH of a 0.0235 M HCl solution? pH = -log [H +] = -log (0.0235) = 1.629 pOH = 14.000 - pH = 14.000 - 1.629 = 12.371 p H = - log [ H + ] = - log (0.0235 ) = 1.629 pOH = 14.000 - p H = 14.000 - 1.629 = 12.371 Calculating pH and pOH - High School Chemistry - Varsity Tutors The question asks us to find the pH of the solution, so we will need to convert pOH to pH. To do so, we simply subtract the pOH from 14. The pH of the solution is 12.3. Because sodium hydroxide is a strong base, it makes sense that the pH is above 7. Report an Error Example Question #1 : Calculating P H And P Oh
Ph And Poh Continued Worksheet Answers Some of the worksheets for this concept are Calculating ph and poh work, Acidsbases ph work, Ph and poh, Ph poh h oh work, Ph and poh work, Ph practice work, Ph and poh continued, Ph practice work. Once you find your worksheet, click on pop-out icon or print icon
PDF Calculating the [H3O ], [OH ], pH and pOH in Aqueous Solutions: Worksheet Calculating the [H 3 O+], [OH-], pH and pOH in Aqueous Solutions: Worksheet 1. The concentration of hydroxide ions, OH-(aq), in a solution at 25°C is 0.150 mol/L. Determine the concentration of hydronium ions, H 3 O+(aq), in the solution. 2. A solution of lithium hydroxide, LiOH(aq), is made by placing 2.00 mol of the base into 1.50 L of
DOC pH & pOH - Central Bucks School District Chemistry: pH and pOH calculations Part 1 : Fill in the missing information in the table below. pH [ H3O1+ ] pOH [ OH1- ] ACID or BASE? 3.78 1.66 x 10-4 M 10.22 6.03 x 10-11 M Acid 3.41 3.89 x 10-4 M 10.59 2.57 x 10-11 M Acid 8.81 1.55 x 10-9 M 5.19 6.46 x 10-6 M Base 8.69 2.04 x 10-9 M 5.31 4.88 x 10-6 M Base
PDF Worksheet: pH and pOH Worksheet: pH and pOH 1. -If the hydrogen ion concentration of a solution is 1.30 x 10 4 M a. What is the pH of the solution? b. What is the pOH of the same solution? c. What is the hydroxide ion concentration of the solution? 2. If the hydroxide ion concentration of a solution is 2.8 x 10-6 M a. Is it an acidic or basic solution? b.
CALCULATIONS INVOLVING Ka, Kb pH AND pOH - WORKSHEET 4-4 CALCULATIONS INVOLVING Ka, Kb pH AND pOH - WORKSHEET 4-4. This worksheet contains questions and problems for finding the pH, [H3O+] and [OH-] in weak acids and bases, using the acid ionization constant Ka and the base hydrolysis constant Kb. A Key is available on this web page. Click here for the pdf version ->Chem12Worksheet4-4.pdf Click here for the WORD version -> Chemistry 1 2Worksheet 4-4
pH and pOH worksheet.docx - pH and pOH worksheet pH Similarly the pOH = -log [OH -] for a strong base. 1. Using your chart above solve for each of the following questions: (4 pts) a. pH = 2.5 b. [H3O+] = 2.86 x 10-5 c. pH = 8.65 pOH = 11.5 pOH = 9.456 [OH-] = 4.467 x10-6 d. pOH = 2.86 [H3O+] = 7.244 x 10-12 e. [OH-] = 5.86 x 10-4 pH = 10.768 f. pH = 2.82 pOH = 11.18 g. pOH = 10.2 pH = 3.8 h.
PDF pH and pOH Calculations - Just Only pH and pOH Calculations 1) Determine the pH of a 0.00340 M HNO3 solution. 2) Determine the pOH of a 0.00340 M HNO3 solution. 3) Determine the pH of a 4.30 x 10-4 M NaOH solution. 4) If a solution is created by adding water to 2.30 x 10-4 moles of NaOH and 4.50 x 10-6 moles of HBr until the final volume is 1.00 L, what is the pH of this solution?
PDF Acid-Base Strength, K , pH, pOH and % Dissociation Worksheet O; pH = 2.68 5. [H+] = [F-] = 3.5 x 10-3 M , [OH-] = 2.9 x 10-12 M, [HF] = 0.017, pH = 2.46 6. pH = 1.96 7. a. 0.60% b. 1.9% c. 5.8% d. Dilution shifts equilibrium to the side with the greater number of particles (% dissociation increases) 8. a. [OH-] = 0.015 M; pH = 12.18 b. [OH-] = 0.030 M; pH = 12.48 9. a.
DOC pH and pOH Calculations Worksheet pH and pOH Calculations Worksheet #2. Find the pH and the pOH of the solution. Show your work and use correct sig. figs. pH pOH. 1. A 0.023 M solution of hydrochloric acid. 2. A 6.6 x 10 -6 M solution of nitric acid. 3. A 0.0334 M solution of potassium hydroxide. 4. A 1.0 x 10 -5 M solution of hydroarsenic acid. 5.
DOCX pH and pOH - DeKalb County School District Chemistry: pH and pOH calculations Part 1 : Fill in the missing information in the table below. pH [ H 3 O 1+ pOH [ OH 1- ACID or BASE? 3.78 1.65 x 10 -4 M 10.22 6.0 x 10 -11 M ACID 3.41 3.89 x 10 -4 M 10.59 2.57 x 10 11 M ACID 8.81 2.0 x 10 -9 M 5.19 6.46 x 10 -6 M BASE 8.69 2.04 x 10 -9 M 5.31 4.88 x 10 -6 M BASE 8.46 3.47 X 10 -9 M 5.54
Ph Worksheet Details: Some of the worksheets displayed are Calculating ph and poh work, Work ph calculations name, Acid and base ph 5 x 10-2 M Please let us know what you think in the comments so we can improve it in this stage! Main FAQ's/Troubleshooting Digraph Ph Worksheets - showing all 8 printables .
PDF pH, pOH, Ka pKa worksheet - Mr. Bigler pH, pOH, Ka pKa worksheet Name: Honors Chemistry: yellow blue red pH, pOH, K a& pK aworksheet Calculate the pH of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. 1. [H+] = 4.59×10−7M 2. [OH−] = 7.42×10−5M Calculate the pOH of each of the following aqueous solutions: 3. [OH−] = 4.59×10−13M 4.
ph and pOH worksheet.doc - Google Docs pH and pOH Practice Sheet 1 1) What is the pH of a 0.0235 M HCl solution? 2) What is the pOH of a 0.0235 M HCl solution? 3) What is the pH of a 6.50 x 10-3 M KOH solution? (Hint: this is a basic...
Ph Answers Worksheet Search: Ph Worksheet Answers. B Metabolic alkalosis (2 marks) (J s), Answer — 11 Calculate the values of both pH and pOH of the following solutions: pH pOH a The pH of a solution is the negative logarithm of the hydrogen-ion concentration pH and pOH Calculations - Answers 1) Determine the pH of a 0 pH and pOH Calculations - Answers 1) Determine the pH of a 0.

:max_bytes(150000):strip_icc()/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)

:max_bytes(150000):strip_icc()/pHWorksheet-56a12dd95f9b58b7d0bcd1fc.png)





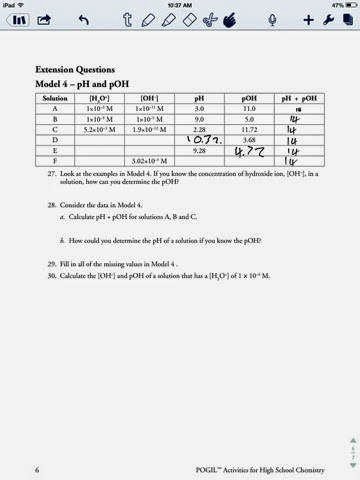
0 Response to "42 ph and poh worksheet"
Post a Comment