40 photoelectron spectroscopy worksheet answers
Spectra Of Elements Worksheet Answers - Google Groups Answer announce the atoms of an element are excited by absorbing the energy from. Bohr's model does not accurately explain the chemical properties of elements. AP Chem HW 3 Photoelectron Spectroscopy Worksheet 1 In a photoelectron spectrum photons of 1657 MJmol strike atoms of an unknown element If the. Photoelectron Spectroscopy - Ultraviolet Photoelectron Spectroscopy ... Photoelectron spectroscopy is a useful technique for scientists because it allows them to explore the properties of matter (gases, solids, and liquids) by measuring electron orbital energies. Scientists can infer particularly about the constituents of a substance as well as the bonds between the atoms in the material using this data.
PDF 1.7 Periodic Trends 1.6 Photoelectron Spectroscopy AP Chemistry 1.5 ... Photoelectron Spectroscopy (you have the POGIL) kJ/mol now MJ/mol The minimum energy needed to remove an electron from an atom or ion in the gaseous state is defined as Ionization energy and the energy required to remove the least tightly held electron is the first ionization energy. Remember Coulomb's law F coulomb 𝝰 q 1 q 2 / r2.

Photoelectron spectroscopy worksheet answers
Photoelectron_Spectroscopy_Worksheet-KEY.docx - Course Hero Photoelectron Spectroscopy (PES) ANSWER KEY 1. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? IE = Energy if Photons - KE of electrons = 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol 2. PDF Name: Date: AP Chemistry Big Idea 1 Worksheet: Photoelectron ... - Heroku 1. Refer to the PES spectrum below. Make note of the relative energies of each peak. a. How do the peak heights compare? What does this tell us about the relative number of electrons represented by each? b. If the peaks shown represent all of the electrons in this atom, identify the element. c. Which peak represents the core (innermost) electrons? PDF PES Electron Configuration - Rancho High School 2. Refer to the PES spectrum below. 100 Binding Energy (M]/ mol) a. How many electrons are represented in each peak? b. How many electrons does the atom contain? How many electrons are in its valence shell? c. Write the electron configuration for this element. C)xqoevx 1.5 Photoelectron Spectroscopy & Electron Configuration 3.
Photoelectron spectroscopy worksheet answers. 50 Photoelectron Spectroscopy Worksheet Answers - Pinterest Photoelectron Spectroscopy Worksheet Answers - 50 Photoelectron Spectroscopy Worksheet Answers , the Triple Point Teaching Resources F Fiveable 242 followers More information Photoelectron Spectroscopy Worksheet Answers Inspirational Ap Chemistry Big Idea 1 Electron Spectroscopy Pes Find this Pin and more on AP Chem by Fiveable. Teaching Science PDF Weebly Created Date: 8/27/2015 7:01:34 AM PDF Photoelectron Spectra Samples - Ms Ingratta's Chemistry Page Photoelectron'Spectroscopy'(PES)Sample'Questions'! ' Questions'113refer'to'the'photoelectron'spectrum'of'neon'shown'below:' ' J. K. Howell R e l a t i v e N u m b e r o f E l e c t r o n s 1000 800 600 400 200 0 Photoelectron Spectrum of Neon Binding Energy (eV) A B C Solved Worksheet: Photoelectron Spectroscopy& Electron | Chegg.com Chemistry questions and answers; Worksheet: Photoelectron Spectroscopy& Electron Contiguration 1. Refer to the PES spectrum below. Make note of the relative energies of each peak 100 Binding Energy (MJ/mol) How do the peak heights compare? What does this tell us about the relative number of electrons represented by each? a. b.
Photoelectron Spectroscopy Pogil Answer Key Pdf Watch The Videos: The Electromagnetic Spectrum And How To ... 811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. answers free download atomic spectra flinn chem topic lab answers''flame test and atomic spectra answer key april 25th, 2018 - flame test and atomic spectra answer key pdf free download here atomic spectra lab answer key answers on ... PDF Photoelectron spectroscopy pogil- answers pdf - Silky Photoelectron spectroscopy pogil- answers pdf ... 3p 2 1s 2 2s 2 2p 6 3s 2 3p 1 1s 2 2s 2 2p 6 3s 2 Worksheet: Electron Configurations Name: More information Why? The chemical properties of an element are based on the number of electrons in the outer shell of its atoms. We use Lewis dot structures to map these valence electrons in order to ... Photoelectron Spectroscopy.docx - Leilani Salgado AP... Leilani Salgado AP Chemistry 9/27/20 Photoelectron Spectroscopy Questions 1. What determines the position and the height (intensity) of each peak in a photoelectron spectrum? The number of ejected electrons vs the corresponding ionization energy for the ejected electrons. Therefore, the peaks determine the electrons in different subshells. PDF Photoelectron Spectroscopy (PES) Worksheet #6 Name: Period: Seat# 5) Below is shown the PES spectrum of sulfur (atomic number = 16) a. Write the full electron configuration of sulfur b. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) On diagram above c. On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic ...
Photoelectron Spectroscopy, Spectrum, PES - AP Chemistry Worksheet Practice This Printable AP Chemistry Worksheet contains carefully selected high-quality multiple-choice questions on Photoelectron Spectrum.A great printable resource to assign to your students for homework, classwork, practice, or review for a quiz, test, or exam.. My resources follow the New AP Chemistry Course Framework.. This worksheet has 34 multiple choice questions in the following two topics of Photoelectron Spectroscopy - AP Chemistry - Varsity Tutors Possible Answers: Correct answer: Explanation: The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1 s peak, it has twice the electrons (4). PDF AP C6 PES problems from CB - DHS 7. Given the photoelectron spectrum of Scandium above, which of the following best explains why Scandium commonly makes a 3+ ion as opposed to a 2+ ion? a. Removing 3 electrons releases more energy than removing 2 electrons. b. Scandium is in Group 3, and atoms only lose the number of electrons that will result in a noble gas electron ... PDF AP WORKSHEET 01g: Photoelectron Spectroscopy (a) Write the electron configuration and identify the element. (2) (b) The plot is divided into three separate areas on the x-axis. Why is the axis divided in this manner? (2) (c) What would be the charge on an ion formed from this atom? Justify your answer. (2) (d) What is the significance of three of the peaks having the same height? (2)
PDF Photoelectron Spectroscopy Worksheet - DePauw University Forn= 1 thereisasinglesubshell,whichwe identifyas1s;forn= 2 therearetwosubshells,whichweidentifyas2sandas2p. Thisallowsustowrite anelectronconfigurationthatdescribesanelement'selectrons. Giventhattheseelectronconfigurationfor lithiumis1s22s1,writetheelectronconfigurationforfluorine. Giventhedataavailabletoyou,howmany electronscanashelllabeledshold?p?
PDF PHOTOELECTRON SPECTROSCOPY - Pinchas's chemsite Photoelectron Spectra for Potassium 1. Place "relative intensity" values on the y axis in the tables above. 2. Label each peak on the graphs above with the subshell each represents (1s, etc). 3. Which electron will be removed from a neutral atom of potassium to form K+? _______________ Explain, using the information provided by the above spectrum:
PDF Ap worksheet 01g : photoelectron spectroscopy worksheets answers (2) (e) The peaks at 1.25 & 2.44, as well as the peaks at 20.2 & 26.8, are relatively close to one another but have different energies? (3) (c) Using your answer in (b), identify the mostly likely charge on an ion of this element.
PDF 1.6 Photoelectron Spectroscopy AP Chemistry 1.5 Atomic Structure Day 5 ... Photoelectron spectroscopy (PES) allows scientists to determine the ionization energy of not only valence electrons, but all electrons in the atom. In PES, a gaseous sample of atoms is bombarded by X-rays or ultraviolet light (photons) of known energy. The kinetic energies of the photoelectrons that are ejected from the atoms are measured.
Quiz & Worksheet - Photoelectron Spectroscopy | Study.com Photoelectron spectroscopy is a very useful lab technique and its data can be used in a variety of ways. Test your understanding of photoelectron spectroscopy with this quiz/worksheet combo. The ...
Practice Quiz - Photoelectron Spectroscopy - ScienceGeek.net Photoelectron Spectroscopy. Click the "Start Quiz" button to proceed ...
PDF Photoelectron Spectroscopy Worksheet - Announcements 1. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol 2.
PDF PES Electron Configuration - Rancho High School 2. Refer to the PES spectrum below. 100 Binding Energy (M]/ mol) a. How many electrons are represented in each peak? b. How many electrons does the atom contain? How many electrons are in its valence shell? c. Write the electron configuration for this element. C)xqoevx 1.5 Photoelectron Spectroscopy & Electron Configuration 3.
PDF Name: Date: AP Chemistry Big Idea 1 Worksheet: Photoelectron ... - Heroku 1. Refer to the PES spectrum below. Make note of the relative energies of each peak. a. How do the peak heights compare? What does this tell us about the relative number of electrons represented by each? b. If the peaks shown represent all of the electrons in this atom, identify the element. c. Which peak represents the core (innermost) electrons?
Photoelectron_Spectroscopy_Worksheet-KEY.docx - Course Hero Photoelectron Spectroscopy (PES) ANSWER KEY 1. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? IE = Energy if Photons - KE of electrons = 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol 2.


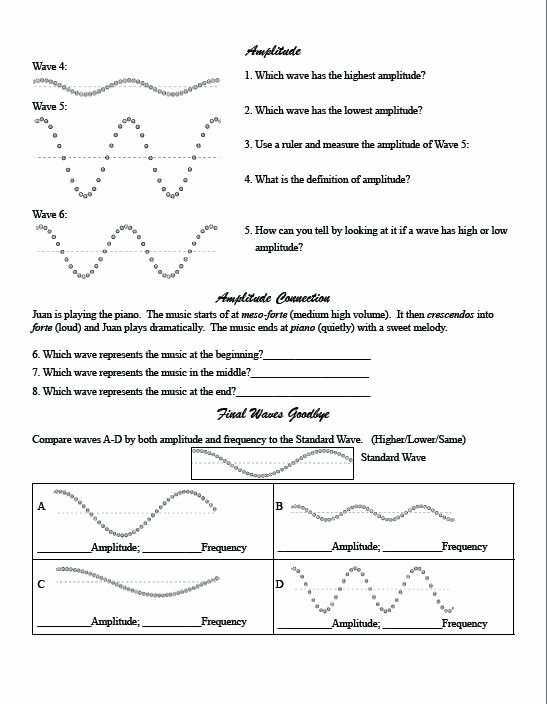
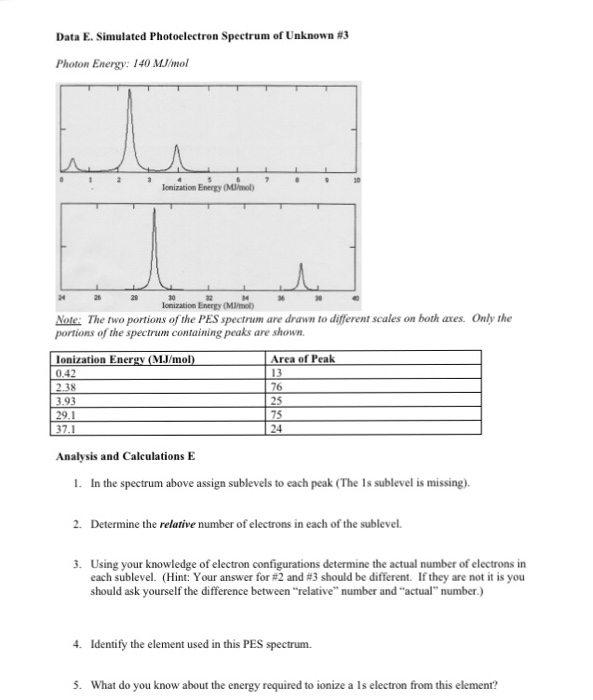
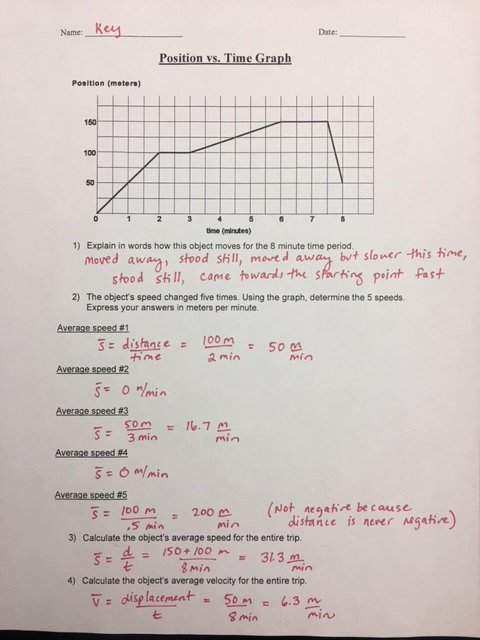

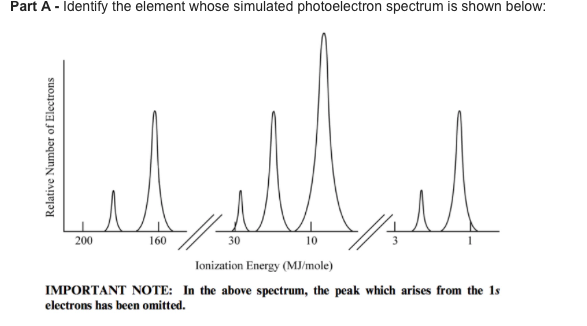



0 Response to "40 photoelectron spectroscopy worksheet answers"
Post a Comment