39 emission spectra and energy levels worksheet
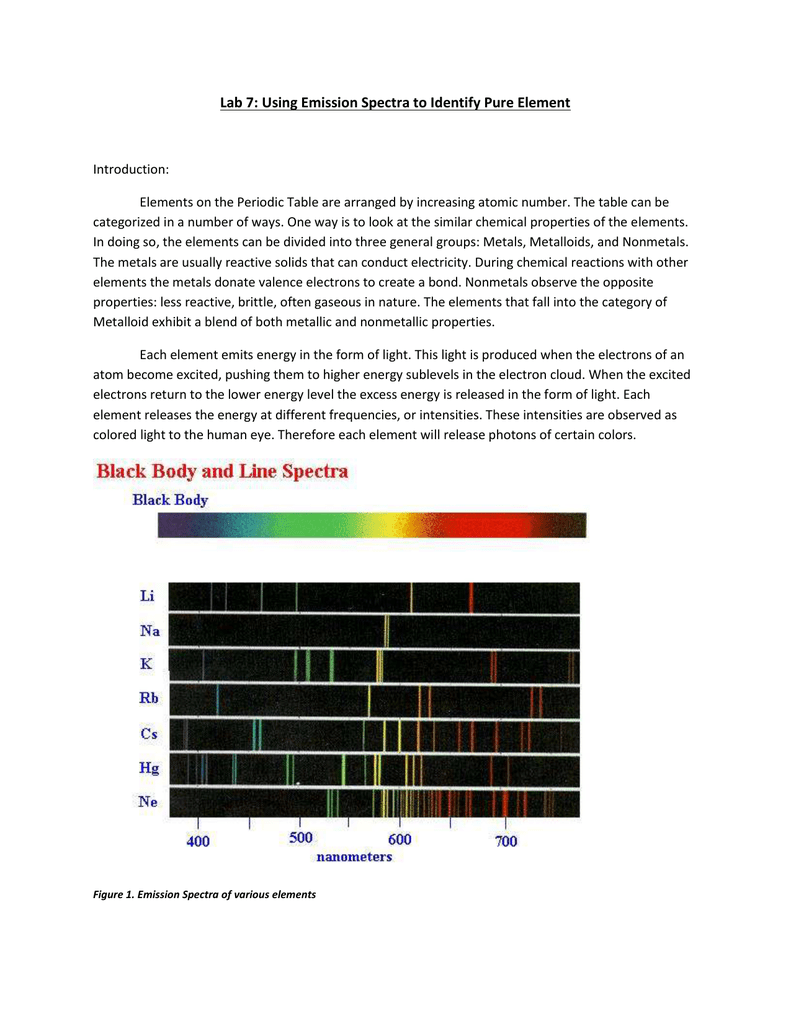
filmreview.us Relative atomic mass of Q _____ Element Q _____ (3) In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. emission spectra and energy levels worksheet answers16 Best Images of Atomic Spectra Worksheet - Electromagnetic Spectrum Worksheet Answers, Atomic from www. EmissionSpectraEnergyLevelsPractice.pdf - Name Date Pd ... movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? The thickness (brightness) depends on the number of photons.
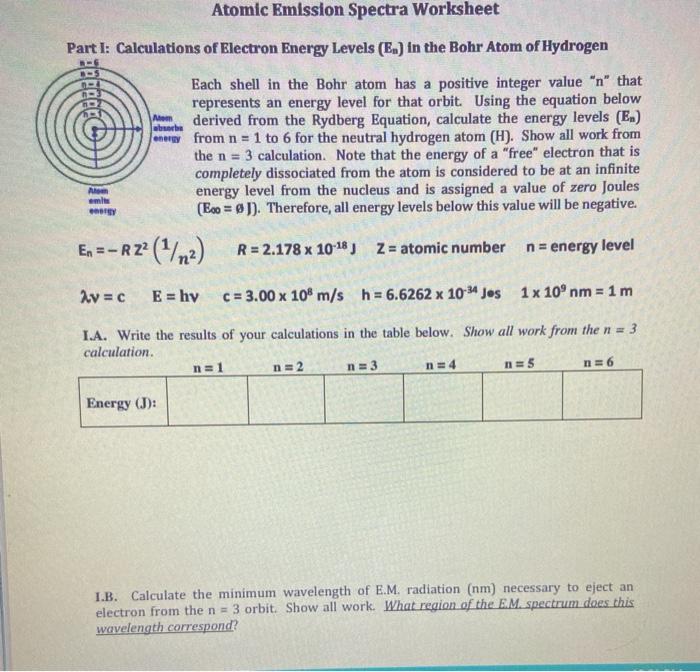
Solved Atomic Emission Spectra Worksheet Part I: | Chegg.com Atomic Emission Spectra Worksheet Part I: Calculations of Electron Energy Levels (E.) in the Bohr Atom of Hydrogen bebe Each shell in the Bohr atom has a positive integer value "n" that represents an energy level for that orbit.

Emission spectra and energy levels worksheet
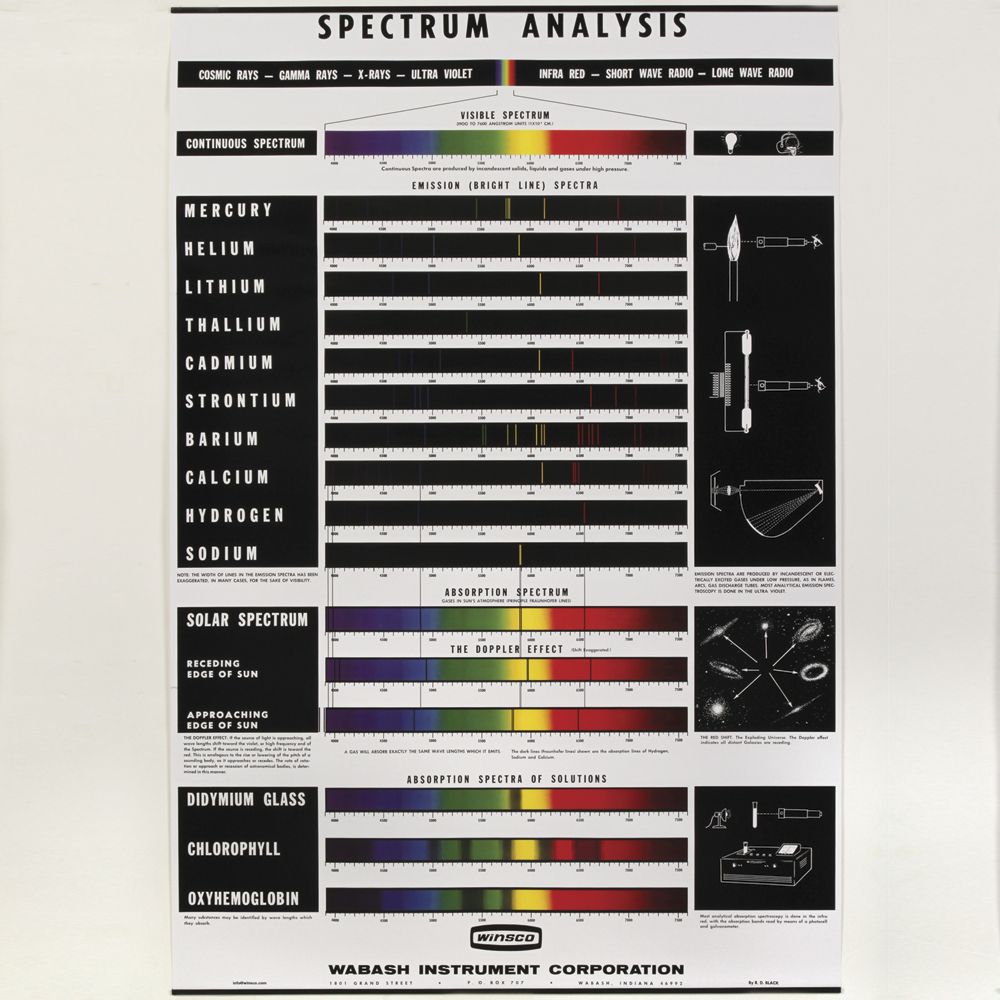
Spectra Of Elements Worksheet Answers The emission spectrum consists of discrete lines corresponding to the differences in energy levels characteristic of and unique along the atoms of the element. Emission Spectra of 10 Elements Answer Key p5 Have students examine the. Photon of radiation that shows up bow this range line-emission spectrum. Worksheet 1 contains the spectra for 7 ... Spectra and energy levels | Teaching Resources A series of discussions and demonstrations looking at spectra and emission levels. Tes classic free licence. Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch. £0.00. Emission spectra and energy levels worksheet pdf - United ... Worksheet 1.3 Emission spectra and electron configurations Each wavelength can be mathematically related to a definite quantity of energy produced by the movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom.
Emission spectra and energy levels worksheet. ##VERIFIED## Emission Spectra Lab Worksheet Answers on ... ##VERIFIED## Emission Spectra Lab Worksheet Answers Sep 27, 2005 — The key is to spread the light out by color, producing a spectrum like the one ... 1. This lab explores some of the basic ideas used to analyze spectra. ... Helium: slightly more complex than hydrogen, with one yellow line and a .... Emission Spectra and Energy Levels Worksheet - Studyres Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? 2. According to the modern theory of the atom, where may an atom's electrons be found? 3. How do electrons become "excited"? 4. Types of Spectra Flashcards | Quizlet Types of Spectra. continuous, emission, absorption. Continuous spectrum. solid or liquid gas heated to incandescence - almost perfect. "Hot dense light source" "core of a star". emission spectrum. black background, single band of light. warm or hot electrons, will only see the colors of light emitted by the atoms. wiens law. Lesson Worksheet:Atomic Emission Spectroscopy | Nagwa In this worksheet, we will practice explaining the emission of fixed colors of light by metal atoms and using line spectra in identifying elements. Q1: The atomic emission spectra of sodium and thallium are shown below. When placed into the flame of a Bunsen burner, sodium gives a vivid yellow flame.
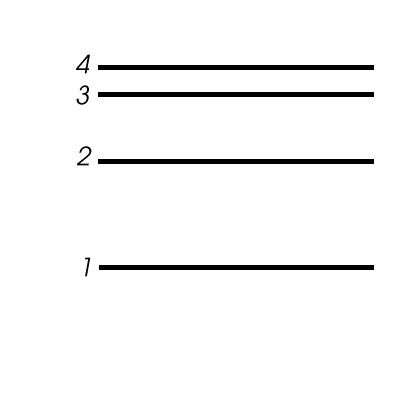
Classwork and Homework Handouts - Penfield Central School ... Major Electron Level Notation Worksheet (DOCX 24 KB) Major Energy Levels (M. E. L.) of electrons Worksheet (DOC 24 KB) Practice with Major Energy Level Configurations Worksheet (DOCX 16 KB) Shell Diagrams of Electrons Worksheet (DOC 37 KB) Spectrum, Electron & Energy Levels Worksheet (DOCX 13 KB) Video - The Wave Mechanical Model Worksheet ... filmreview.us Oct 02, 2013 · Each line is one unit. unit worksheet clearly explain what is meant the term ans) refraction is the bending of light as it passes across the boundary between two different Constant Velocity Particle Model Worksheet 2: shown below at left. J of heat are applied to 10. A kilometer is equal to 1,000 meters, and a kilogram is equal to 1,000 grams. Analyzing Spectra - Weber State University Consider just four of the energy levels in a certain atom, as shown in this diagram: Assume an emission spectrum and draw arrows indicating the possible transitions. How many spectral lines will result from all possible transitions among these levels? Which transition corresponds to the highest frequency (shortest wavelength) light emitted? Chemistry Quizzes - Study.com Interested in seeing how well you know a particular chemistry concept? Take Study.com's brief multiple-choice quiz. Obtain rapid feedback and results to …
2 Emission Spectra Energy Levels and Spectral Charts-1.pdf ... Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? 2. According to the modern theory of the atom, where may an atom's electrons be found? 3. How do electrons become "excited"? 4. Pre-lab: Emission Spectra and Energy Levels You'll ... Start studying Pre-lab: Emission Spectra and Energy Levels. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Lesson Worksheet:Emission and Absorption Spectra | Nagwa In this worksheet, we will practice determining the composition of a material from the features that appear in the spectrum of light coming from it. Q1: A scientist has a sample of an unknown gas. In order to identify it, she shines a continuous spectrum of white light through the gas and observes which wavelengths of light are absorbed by it. Quiz & Worksheet - Line Emission Spectra | Study.com Worksheet 1. Which of the following best describes the reason that line emission spectra contain lines? The energy levels in an atom are discrete and only certain emission wavelengths are possible....
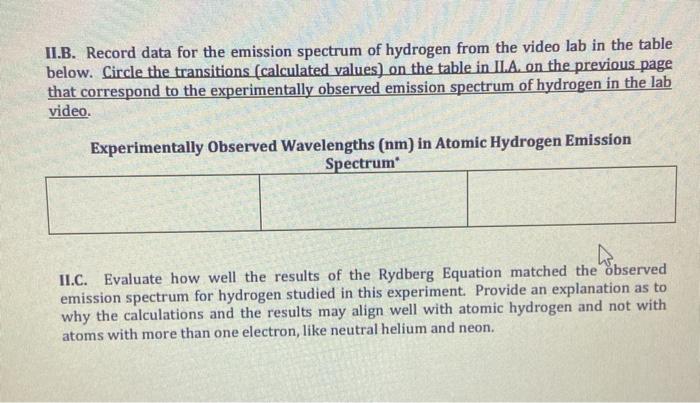
PDF Emission Spectra and Energy Levels energy produced by the movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist at definite, distinctive energy levels in an atom. Problem: In this experiment, you will study the emission spectra of three elements: hydrogen, neon, and helium. You will
virtual-mode.de PT101 Academic English - Placement Test for Secondary SCH3U Chemistry 11. )More SCH4U SCH4U Chemistry: Questions & Answers. It includes 10 worksheets, with answers, and is 27 pages Learning Outcomes: Interconvert energy units. This is a Worksheet Package for the Orbital and Bonding unit of Grade 12 Chemistry. 1 Page (4) cVim Keyboard Shortcuts.
PDF Worksheet 1.3 Emission spectra and electron configurations No. Question Answer 1In which region of the electromagnetic spectrum would you find lines of the greatest energy—ultraviolet, visible or infrared? 2On the energy level diagram show the electron transitions that would result in the series of lines that are seen in the visible region of the hydrogen emission spectrum.
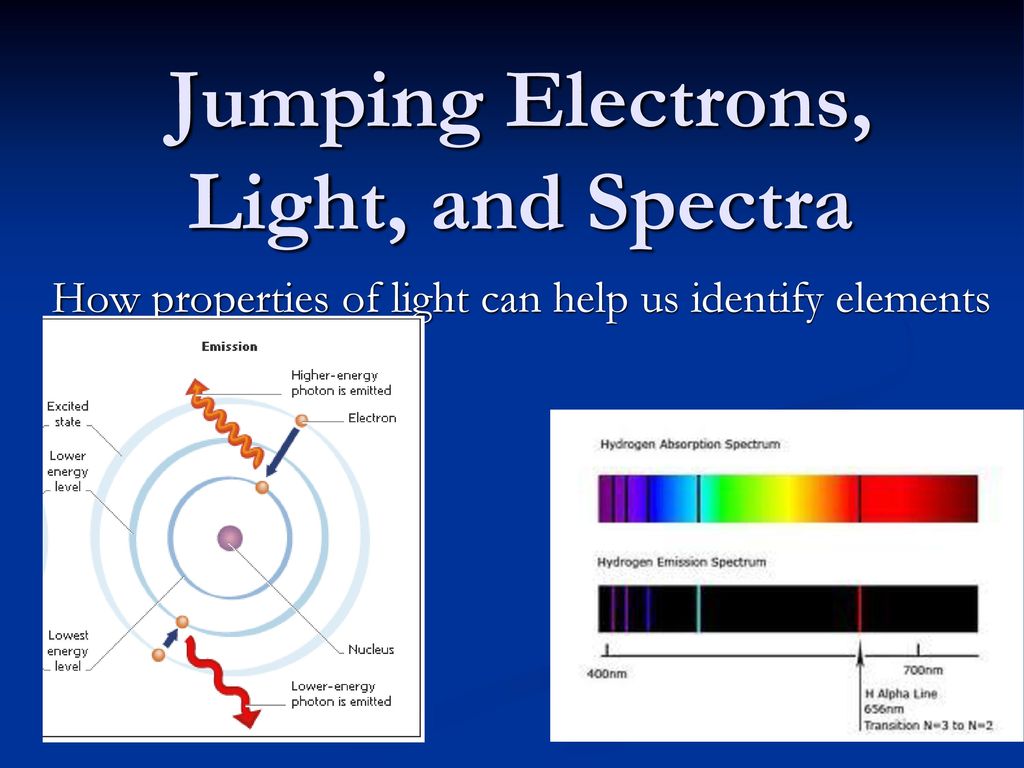
PDF Atomic Emission Spectra Atomic Emission Spectra The electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The ground state of an atom is the lowest energy state of the atom. When those atoms are given energy, the electrons absorb the energy and move to a higher energy level.
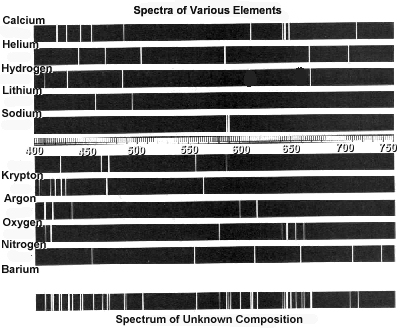
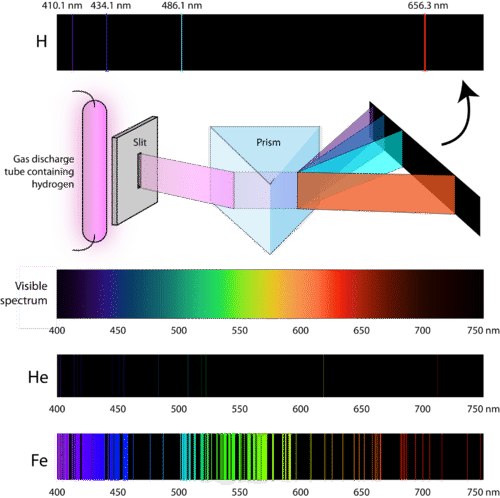
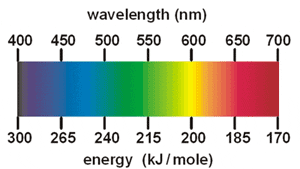
Student Worksheet: Graphing Spectra - NASA Hydrogen. We can identify three bright lines for hydrogen in the top spectrum. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). Recall (e.g. from the Calculation Investigation) that the the frequency is given by n = c / l , and the energy is given by E = hn (where h = 6.626 x 10 -34 J-s, and c = 3 x ...
PPTX PowerPoint Presentation A quantum of energy in the form of light is emitted when the electron drops back to a lower energy level. 5.3 An Explanation of Atomic Spectra The light emitted by an electron moving from a higher to a lower energy level has a frequency directly proportional to the energy change of the electron. 5.3 An Explanation of Atomic Spectra
Chemistry 101 8-ATOMIC EMISSION SPECTRA excited absorb ... Chemistry 101 8-ATOMIC EMISSION SPECTRA. Knowledge of the arrangement of electrons around the nuclei of atoms has been obtained by examining the light emitted by excited atoms.Atoms become excited when they absorb energy; they then emit energy in the form of light as they return to a less excited state.Under
germany-community.de Chapter 9 chapter assessment applying scientific methods answers
Thank you for your interest in - wohnart-coesfeld.de Make observations and measurements to identify materials based on their properties. 3 Emission and absorption spectra | Optical phenomena K-8 Texas Amplify Science Grade 4 Unit 1- Energy Conversions Chapter 1 (Lessons 1 -6)All activities, questions, and answers come directly from the program in the order it's written in the teacher guide ...
Emission Spectra Worksheets & Teaching Resources | TpT This activity worksheet, reviews the emission spectrum of hydrogen including the electromagnetic spectrum, where students identify frequency, energy and wavelength. Labeling and describing absorption spectra and emission spectra, including a continuous spectrum, to identify the differences between t Subjects: Chemistry, Physical Science, Physics
Four Quantum Numbers: Principal, Angular Momentum ... Aug 18, 2021 · Quantum numbers describe specific properties of an electron. Learn about atomic orbital, the four quantum numbers (principal, angular momentum, magnetic, and spin), and how to write quantum ...
PDF Flame Test Lab Activity Key - University of South Florida wavelength of 459 nm. What is the energy content, in joules, of this photon? (-34 8)( )-19-7-19 6.33 x 10 J s 3.00 x 10 m s = = 4.33 x 10 J 4.59 x 10 m = 4.33 x 10 J E E ⋅ 3. Emission spectrums are produced from the release of light by electrons in varying energy levels. The following is an emission spectrum of hydrogen gas. Calculate the
PDF X-ray Spectroscopy and the Chemistry of Supernova Remnants ... Student Worksheet: Graphing Spectra Part 2 The following spectrum represents the energy state of the element, carbon. Carbon's emission lines in the visible range are a function of wavelength from 4,000 to 7,000 Ångstroms. You are going to create a graphical representation of carbon's spectrum from the photographic representation.
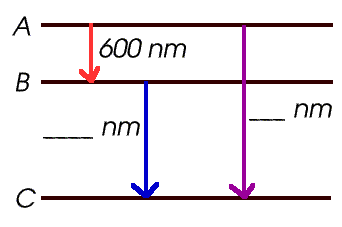
Energy Levels and Spectra - Physics Things Calculate the energy difference (in Joules) between energy levels. Using either E = hf, E = hc/wavelength or for electrons E = 1/2mv^2 you can work out the energy of the incident photon/electron. If this energy is equal to the difference in energy levels, the electron will be excited.
Emission spectra and energy levels worksheet pdf - United ... Worksheet 1.3 Emission spectra and electron configurations Each wavelength can be mathematically related to a definite quantity of energy produced by the movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom.
Spectra and energy levels | Teaching Resources A series of discussions and demonstrations looking at spectra and emission levels. Tes classic free licence. Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch. £0.00.
Spectra Of Elements Worksheet Answers The emission spectrum consists of discrete lines corresponding to the differences in energy levels characteristic of and unique along the atoms of the element. Emission Spectra of 10 Elements Answer Key p5 Have students examine the. Photon of radiation that shows up bow this range line-emission spectrum. Worksheet 1 contains the spectra for 7 ...





















0 Response to "39 emission spectra and energy levels worksheet"
Post a Comment