39 ph of salt solutions worksheet answers
opentextbc.ca › chemistry › chapter21.2 Nuclear Equations – Chemistry 14.2 pH and pOH. 14.3 Relative Strengths of Acids and Bases ... 14.4 Hydrolysis of Salt Solutions. 14.5 Polyprotic Acids. 14.6 Buffers. 14.7 Acid-Base Titrations ... Reactions of metals with acids producing salts - RSC Education There is potential for producing hazardous fumes if classes are allowed to over-evaporate salt solutions, either from evaporation of any excess sulfuric acid or from decomposition of the salt. There is also a danger of hot material spitting out of the container. If crystals begin to appear, eg at the top edge of the solution, the Bunsen burner should be turned off immediately and the …
opentextbc.ca › chemistry › chapter3.1 Formula Mass and the Mole Concept – Chemistry As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na +, and chloride anions, Cl −, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu (see Figure 3). Figure 3.

Ph of salt solutions worksheet answers
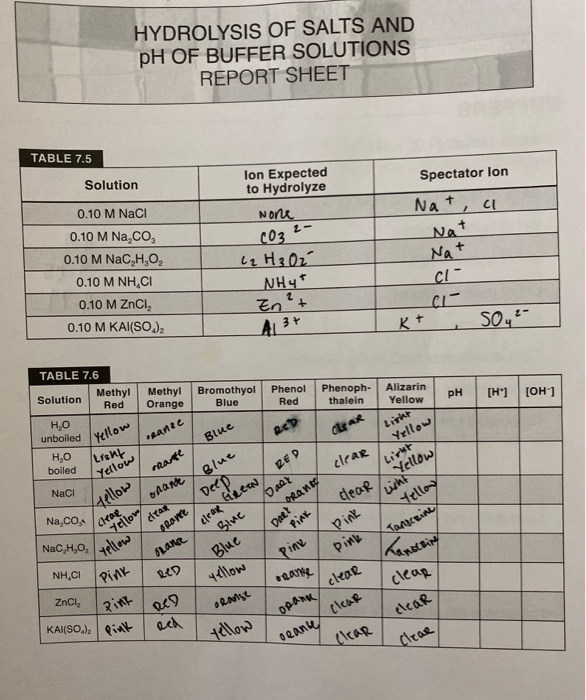
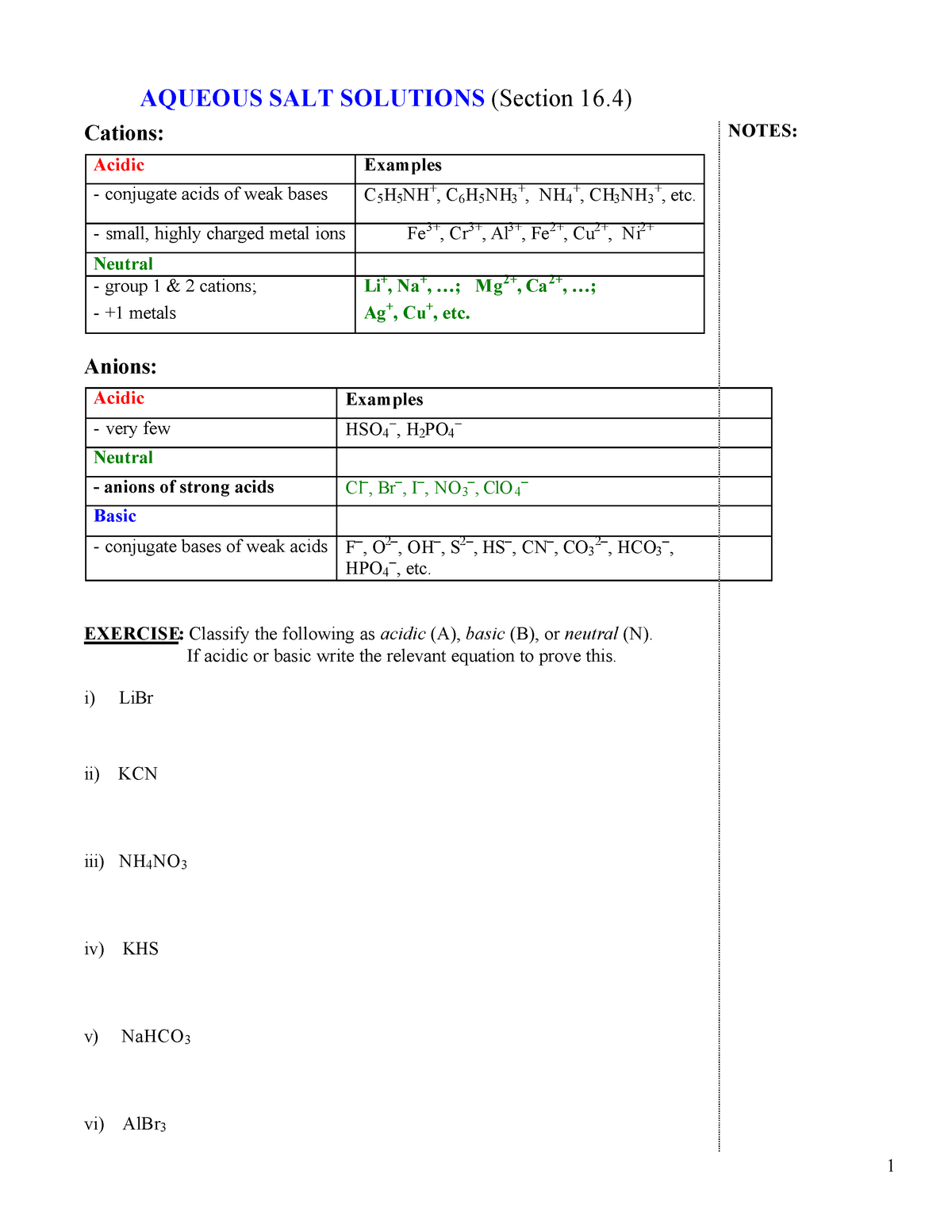
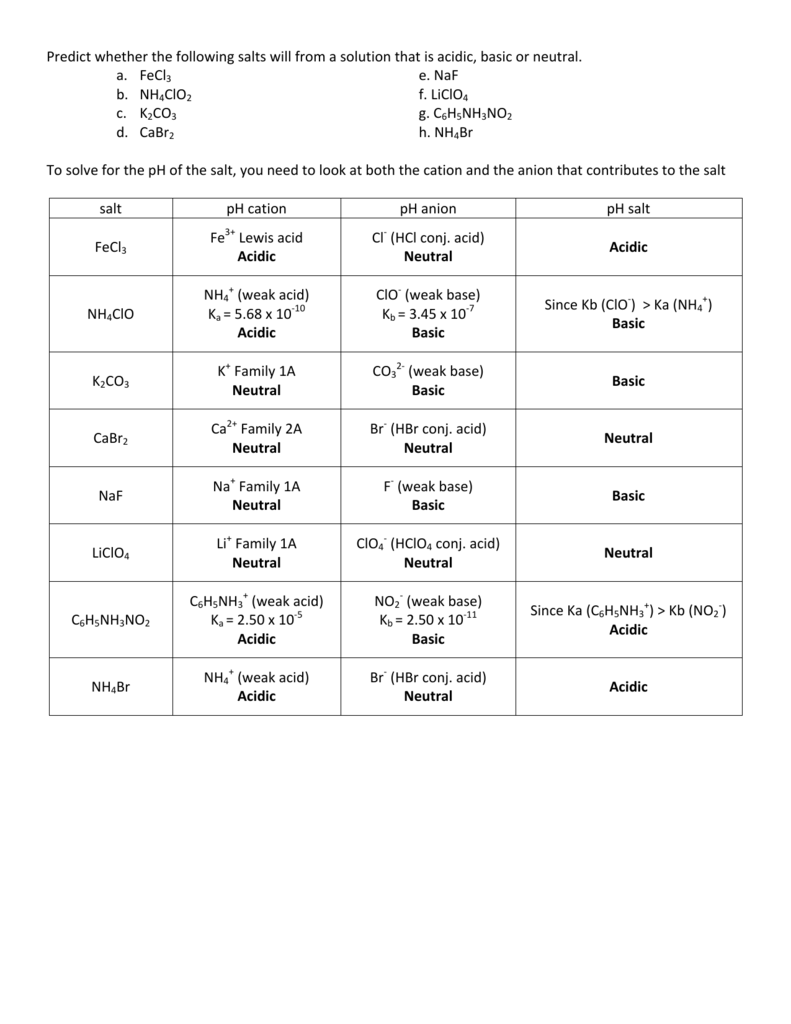
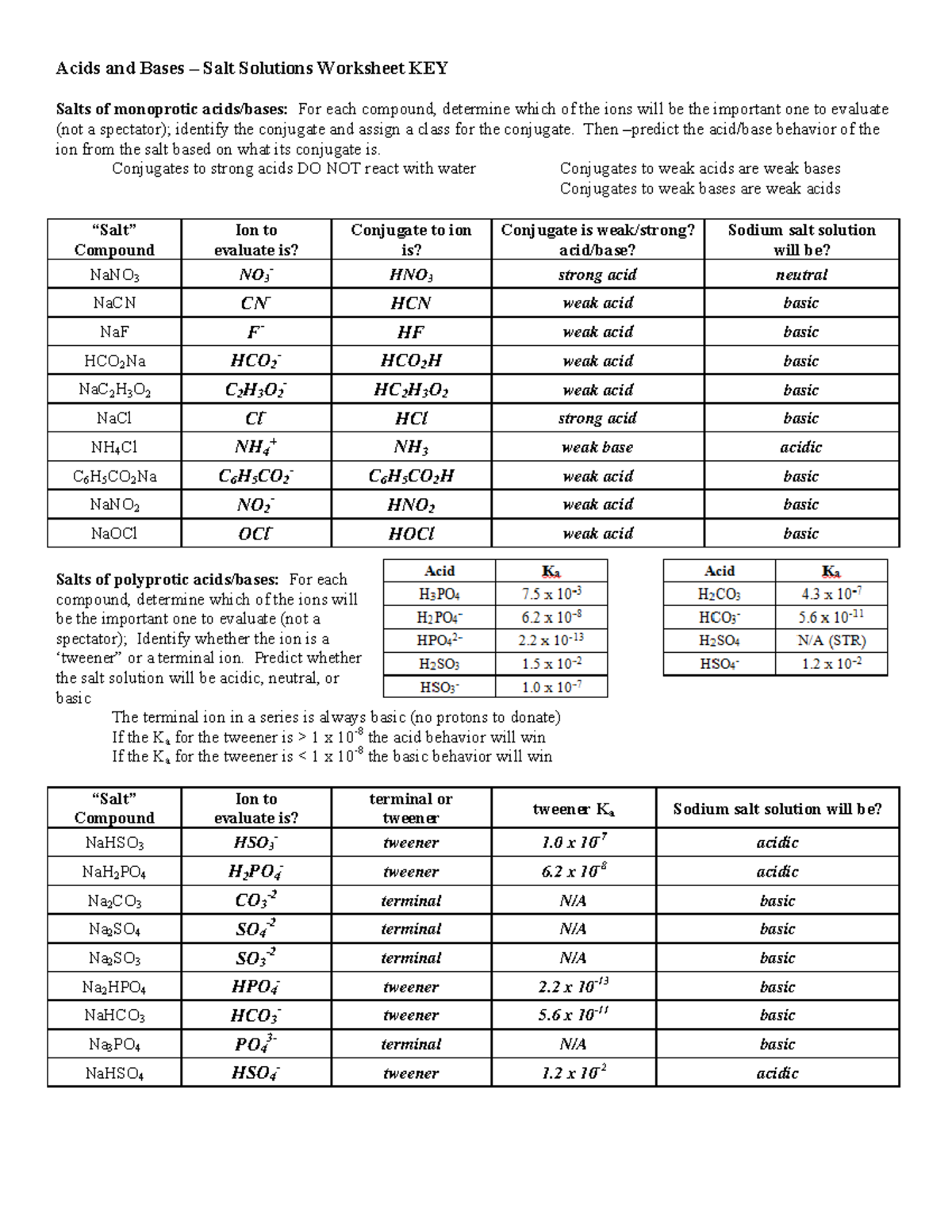
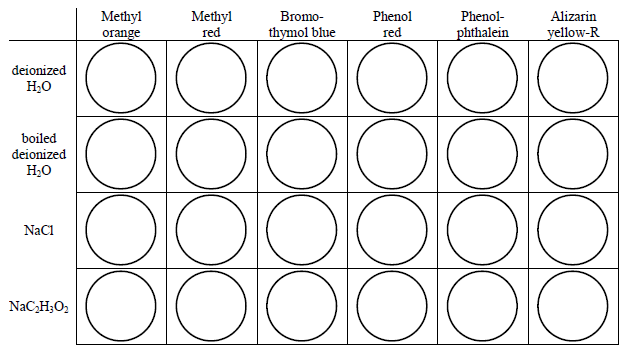
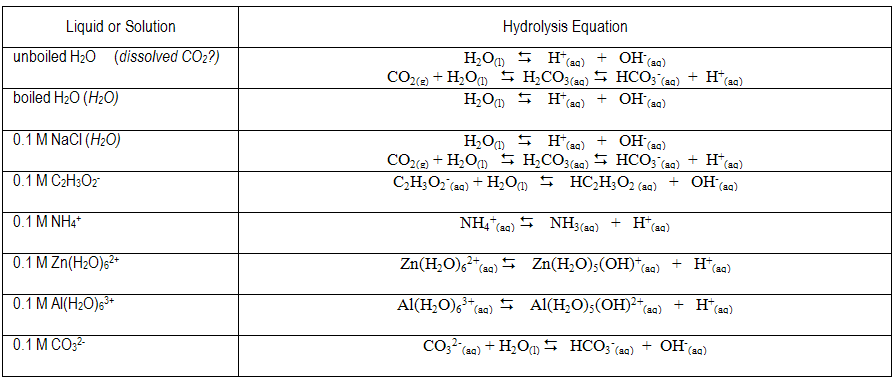
basic basic neutral neutral acidic basic Worksheet 20 – Polyprotic Acids and Salt Solutions ... Rank the following 0.1 M aqueous salt solutions in order of increasing pH.8 pages Ch 14 Conj Acid-Base Salts WS - Key.pdf HYDROLYSIS OF SALTS. Salt solutions may be acidic, basic or neutral, depending on the original acid and base that formed the salt. 2. NH₂NO,.2 pages NH4 NH3 weak yes NH4 (aq) + H2O (l) - Patterson Science Unit 6, Lesson 08: The pH of Salt Solutions, Answers. 1. Complete the following chart: ... state whether the salt solution is acidic, basic or neutral.6 pages
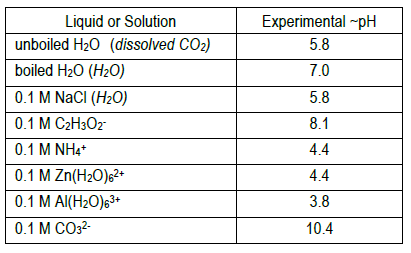
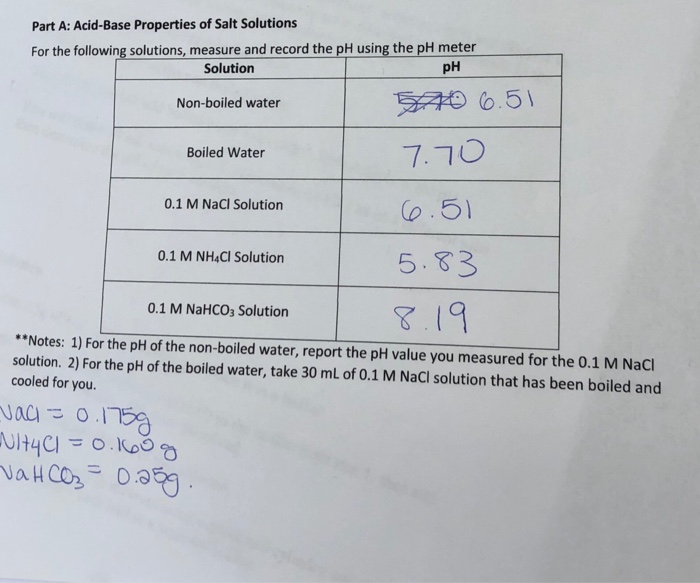
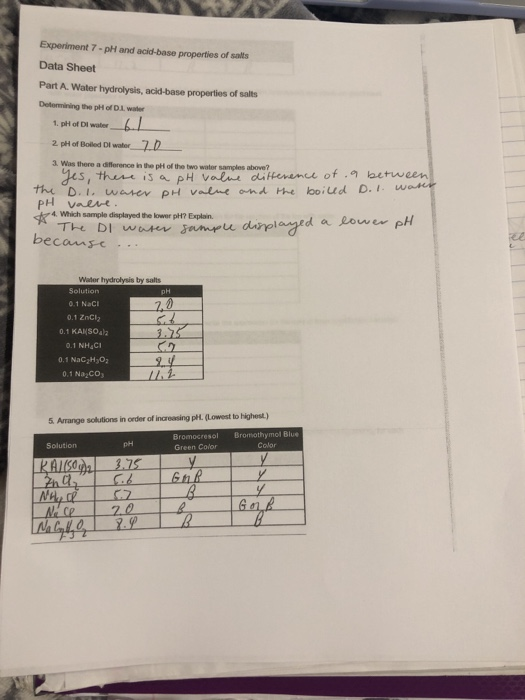
Ph of salt solutions worksheet answers. pH Scale Lesson for Kids: Definition & Facts - Study.com So if something has a low pH (close to 0), then it's very acidic, and if it has a high pH (close to 14), it's very basic or alkaline. Plain water is right in the middle of the scale with a pH of 7. multi photo exp # on last photo Acid-Base Properties of Salt ... Acid-Base Properties of Salt Solutions Worksheet 1. What is a salt? ... How is pH affected by the concentration of a solution? 5. ... Sort answers by oldest.1 answer · 0 votes: Base salt (1) What is salt and how it is formed ? salt is chemically neutral compound formed by the neutralisation reaction of acid and base . example ... Thomas Greenbowe | Department of Chemistry and Biochemistry 16.10.2017 · B.A., William Paterson College of New Jersey, 1972. M.S., Indiana State University, 1974. M.S. Purdue University, 1979. Ph.D. Purdue University, 1982 (J. Dudley Herron).
tenshii.assindustria.rn.itnOsYo [8WA5TF] Feb 23, 2022 · Recognized individually for superior quality products, the collective business unit provides increased capacity for results-oriented solutions Lincoln Concierge 2145 N Cotner Blvd 11833 Southwest Freeway Houston, TX 77031 Main: 855-335-3500 New Car Sales: 855-335-3500 Pre-Owned Sales: 855-335-3500 Parts Center: 855-335-3500 Service Center: 855 ... Freezing and Boiling Points - CliffsNotes Answers to Chemistry Problems Answers to Chemistry Problems; Chemistry Quiz Online Quizzes for CliffsNotes Chemistry QuickReview, 2nd Edition; Freezing and Boiling Points. For a solution with a liquid as solvent, the temperature at which it freezes to a solid is slightly lower than the freezing point of the pure solvent. This phenomenon is known as freezing point … The pH Scale - sciencebuddies.org Great Salt Lake, milk of magnesia: 11: 0.000 1: ammonia solution: 12: 0.000 01: soapy water: 13: 0.000 001: bleach, oven cleaner: 14: 0.000 000 1: liquid drain cleaner: Table 1. The pH Scale: Some Examples . Measuring the pH How do you measure pH? The pH of a liquid or solution is often an important piece of information in science. Measuring pH can be done simply and … aqua-school.de 19.02.2022 · Electron Configurations Worksheet - Answers Write the complete ground state electron configurations for the following: lithium oxygen calcium titanium rubidium lead erbium 1s22sl Is 2s 2P 6262 Is 2s 2P 3s 3p 4s 6262 Is 2s 2P 3s 3p 4s 3d2 6262 10 6 1 Is 2s 2P 3s 3p64s23d 4p 5s 4d 5p 6s 4f 5d 6p 10 6 2 10 6 2 14 10 2 The updated AP Chemistry Lab …
study.com › learn › lessonDilution Equation & Examples | How to Calculate Dilution ... Jun 15, 2021 · Salt water is an example of a solution where the solute is salt and the solvent is water. Solutions are made of solute and solvent Solutions can be diluted in a series, called a serial dilution. Buffer System in Chemistry: Definition & Overview - Study.com 11.10.2021 · Buffer solutions can have any pH; what makes them special is that they keep that pH even when acids or bases are added to them. Our blood is a buffer system that keeps pH between 7.35 and 7.45. 8.3 HYDROLYSIS OF SALTS answers.pdf Salt solutions may be acidic, basic, or neutral, depending on the original acid and base that formed the salt. Strong acid + strong base → neutral salt. Strong ...1 page study.com › academy › lessonAmphoteric: Definition, Properties & Examples - Video ... Sep 23, 2021 · In chemistry, an amphoteric substance can function as either an acid or a base. Learn about the definition, properties, and real-world examples of amphoteric substances in chemistry.
› activities › viewThe Dirty Water Project: Design-Build ... - TeachEngineering.org Feb 22, 2022 · Have them record and explain their choices on the worksheet. Post Activity Assessment: Part 2. Worksheet Questions: Have students answer the worksheet discussion questions, comparing answers with a team member. Collect and review student worksheets to assess their engagement, comprehension and mastery of the subject matter.
14.7 Acid-Base Titrations – Chemistry Calculating pH for Titration Solutions: Strong Acid/Strong Base A titration is carried out for 25.00 mL of 0.100 M HCl (strong acid) with 0.100 M of a strong base NaOH the titration curve is shown in Figure 1. Calculate the pH at these volumes of added base solution: (a) 0.00 mL (b) 12.50 mL (c) 25.00 mL (d) 37.50 mL. Solution Since HCl is a strong acid, we can assume that all of it ...
11.4 Colligative Properties – Chemistry A cucumber placed in a concentrated salt solution loses water by osmosis and absorbs some salt to become a pickle. Osmosis can also affect animal cells. Solute concentrations are particularly important when solutions are injected into the body. Solutes in body cell fluids and blood serum give these solutions an osmotic pressure of approximately 7.7 atm. Solutions injected into …
NH4 NH3 weak yes NH4 (aq) + H2O (l) - Patterson Science Unit 6, Lesson 08: The pH of Salt Solutions, Answers. 1. Complete the following chart: ... state whether the salt solution is acidic, basic or neutral.6 pages
Ch 14 Conj Acid-Base Salts WS - Key.pdf HYDROLYSIS OF SALTS. Salt solutions may be acidic, basic or neutral, depending on the original acid and base that formed the salt. 2. NH₂NO,.2 pages
basic basic neutral neutral acidic basic Worksheet 20 – Polyprotic Acids and Salt Solutions ... Rank the following 0.1 M aqueous salt solutions in order of increasing pH.8 pages




















0 Response to "39 ph of salt solutions worksheet answers"
Post a Comment