38 isotopes and average atomic mass worksheet answers
"Mass Spectrometer" quiz questions and answers PDF: Modern method for separation of isotopes is, with answers for GRE subject tests. 'isotopes and average atomic mass worksheet answer key april 29th, 2018 - the relative atomic mass a r is the mass of one mole of atoms of an Phet Isotopes And Atomic Mass Worksheet Answer Key. a) Does data from … Pre-AP Chemistry: Worksheet #3.3 Isotopes and Average Atomic Mass 1. Name two ways that isotopes of an element differ. Mass Number, Atomic Mass, Neutrons 2. What data must you know about the isotopes of an element to calculate the atomic mass of the element? Atomic Mass of each isotope and % abundance of each isotope 3. The four isotopes of lead are …
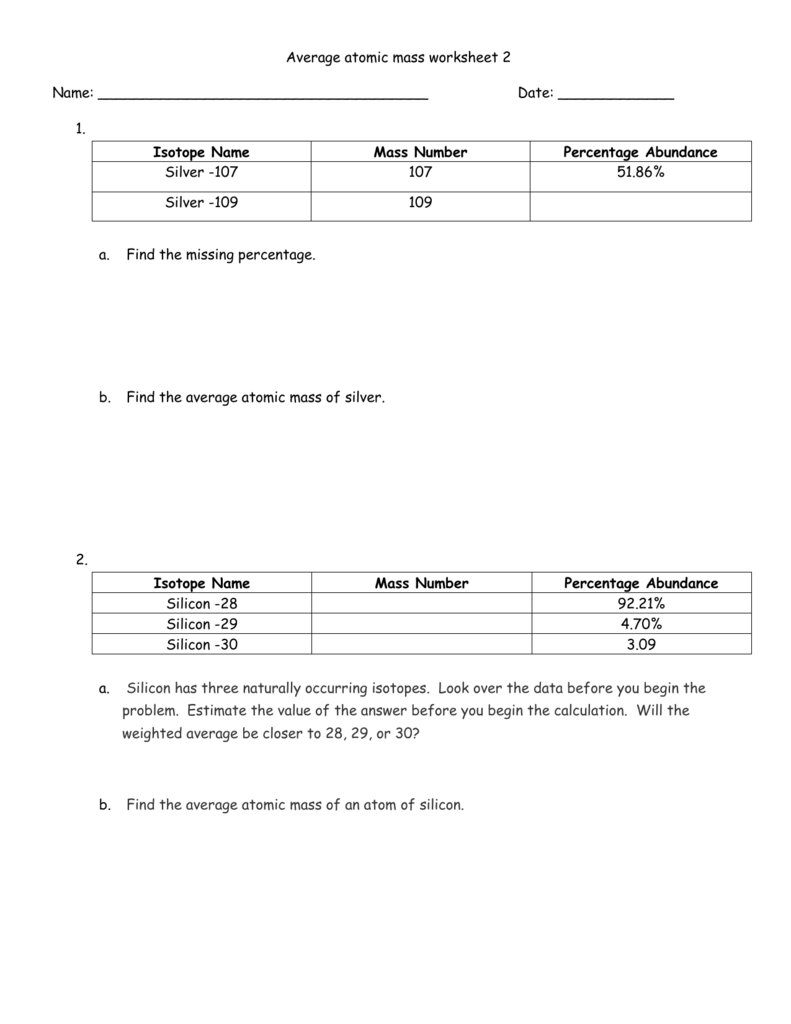
Sample Problem: Calculating Atomic Mass . Use the atomic masses of each of the two isotopes of chlorine along with their percent abundances to calculate the average atomic mass of chlorine. Step 1: List the known and unknown quantities and plan the problem. Known . chlorine-35: atomic mass = 34.969 amu and % abundance = 75.77%

Isotopes and average atomic mass worksheet answers
An atom is made up of protons and neutrons which are in the nucleus, ... The average atomic mass is the weighted average of all the isotopes of an element.4 pages 27 Oct 2019 — Isotopes and Average Atomic Mass. Isotopes are atoms of the same element (they have the same number of protons) but with different.2 pages 2) Uranium is used in nuclear reactors and is a rare element on earth. Uranium has three common isotopes. If the abundance of 234U is 0.01%, the abundance of ...
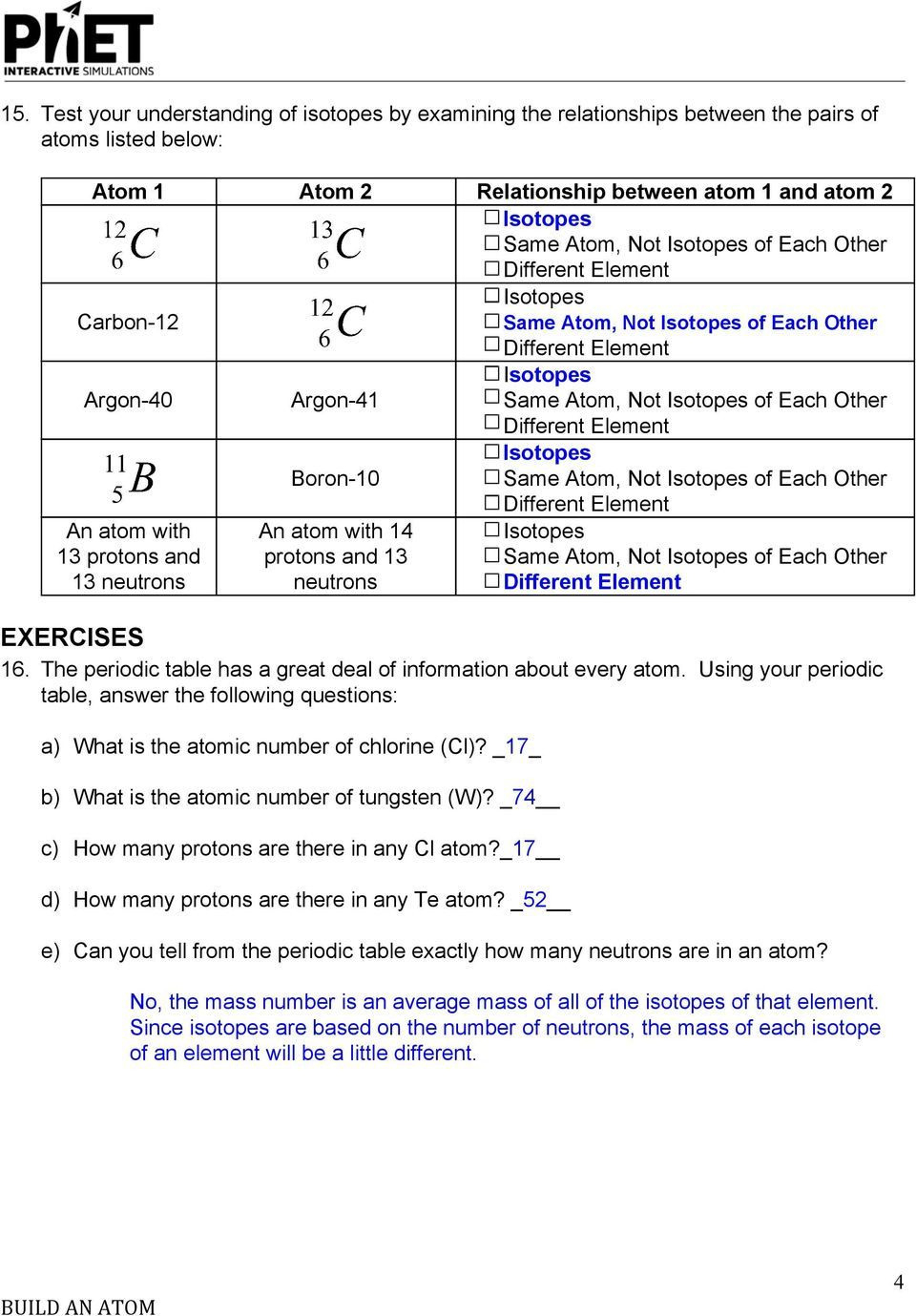
Isotopes and average atomic mass worksheet answers. Calculating Average Atomic Mass Answers Calculating the average atomic mass Yahoo Answers April 7th, 2019 - Need help with a problem Calculate the average atomic mass from the relative abundance and masses of the five naturally occurring isotope of zinc 64 Zn 48 89 63 929amu 66 Zn 27 81 65 926amu 67 Zn 4 11 66 927amu 68 Zn 18 57 67 925amu 70 Zn This … Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. Isotopes and Atomic Mass - PhET Interactive Simulations About This Quiz & Worksheet. There's quite a lot to be learned about atoms. This article and quiz focuses on just some of that, by asking you to recall facts …
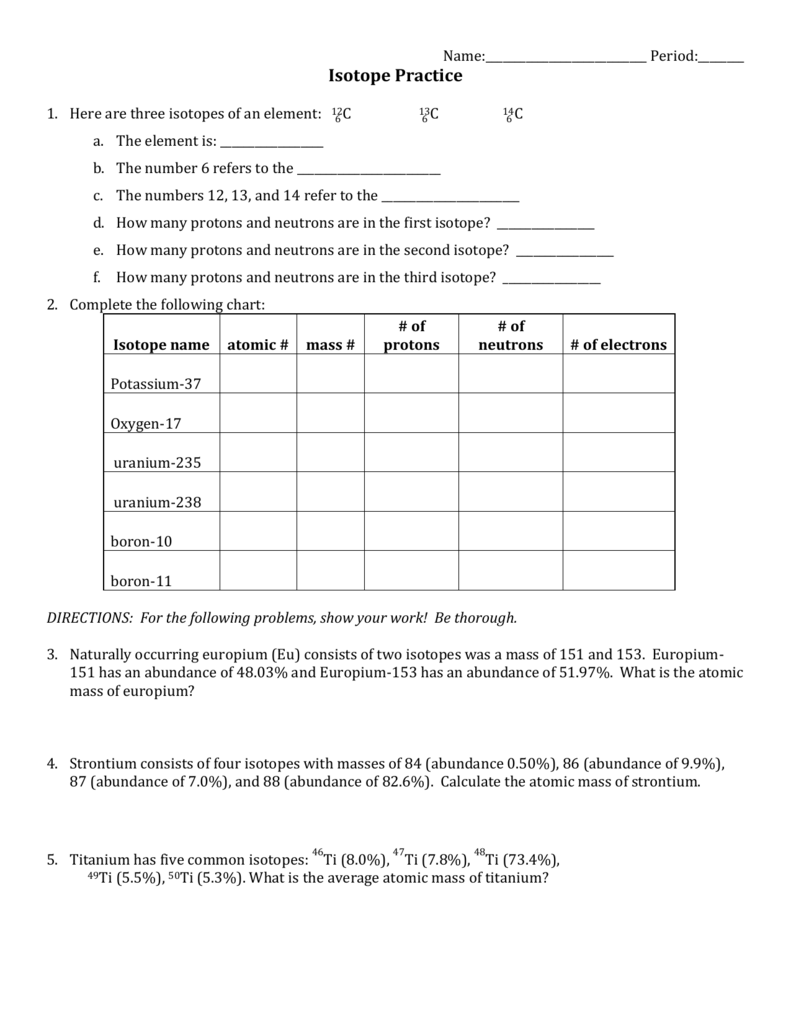
Notice that the answer is closer to 35 than it is to 37. This is because the chlorine-35 isotope is much more abundant than the chlorine-37 isotope. Question. Explain why atoms have different isotopes. In other words, how is it that helium can exist in three different forms? Neutrons exist to stabilize the nucleus ...2 pages Isotope Practice Worksheet 1. Here are three isotopes of an element: 6 12C 6 13C 6 14C a. The element is: Carbon b. The number 6 refers to the atomic number c. The numbers 12, 13, and 14 refer to the mass number d. How many protons and neutrons are in the first isotope? 12 e. How many protons and neutrons are in the second isotope? 13 f. How many protons and neutrons … An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu). Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron) = mass of one carbon-12 atom = 1 amu (atomic mass unit) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson
18 Sept 2020 — Calculate the average atomic mass of this element. Answer ... Bromine has two isotopes, 79Br and 81Br, whose masses (78.9183 and 80.9163 ... The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are: 69.2% for mass of 62.93u . 30.8% fora mass of 64.93u. Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur. if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% . has a mass of 32.971u … 2) Uranium is used in nuclear reactors and is a rare element on earth. Uranium has three common isotopes. If the abundance of 234U is 0.01%, the abundance of ... 27 Oct 2019 — Isotopes and Average Atomic Mass. Isotopes are atoms of the same element (they have the same number of protons) but with different.2 pages
An atom is made up of protons and neutrons which are in the nucleus, ... The average atomic mass is the weighted average of all the isotopes of an element.4 pages

























0 Response to "38 isotopes and average atomic mass worksheet answers"
Post a Comment